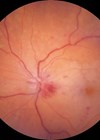
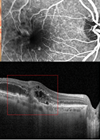
The aim of this study was to investigate initial results regarding the treatment of neovascular glaucoma (NVG) with intravitreal aflibercept. NVG is classified into stages 0-3. In stages 1 and 2, abnormal vessel proliferation is seen with or without elevated IOP, but with an open anterior chamber angle. In stage 3, fibrovascular contraction leads to the development of peripheral anterior synechiae and subsequent secondary angle closure. This study was a prospective interventional case series recruiting patients with newly diagnosed stage 1 or 2 NVG. Four patients were recruited into the study. Each patient received 2mg intravitreal aflibercept at day one with planned additional injections at four weeks, eight weeks and at eight week intervals throughout the study duration (52 weeks). Intravitreal aflibercept resulted in rapid regression of neovascularisation of the iris and angle (NVI, NVA). IOP was stable or reduced in all patients at the final follow-up of 52 weeks. The authors conclude that intravitreal aflibercept may be an effective treatment for stage 1 and stage 2 NVG. The advantage of aflibercept when compared to the traditional treatment of laser panretinal photocoagulation (PRP) for NVG is preservation of the retina, as PRP can cause visual field defects and reduced colour vision and contrast sensitivity. This study is limited by the small sample size and the absence of control subjects and further trials are warranted for the use of intravitreal aflibercept in NVG.