The author explains why an OCT-based classification of neovascular AMD is needed and how these neovascular subtypes may help to predict patients’ long-term visual outcome.
Age-related macular degeneration (AMD) is a chronic and progressive neurodegenerative process involving the macula in elderly patients. The natural history of AMD leads to severe loss of central vision due to variable degrees of atrophy, scarring and chronic exudation [1]. AMD represents a major sociosanitary issue in developed countries given the high prevalence and incidence, and the direct and indirect economic resources related to the management of the disease and its negative vision impact [2].
AMD is classified into different successive stages, ending with advance AMD defined by the presence of foveal involvement by geographic atrophy (GA) of the macular retinal pigment epithelium (RPE) and / or choroidal neovascularisation (CNV) [3]. The neovascular form of AMD used to be also classified into classic and occult lesions according to the angiographic appearance of the neovascular complex in fluorescein angiography (FA) as described by the Macular Photocoagulation Study [4].
Classic neovascular lesions correspond to areas of early well-demarcated choroidal hyperfluorescence with progressive pooling of dye leakage in the overlying subretinal space in the later phases of the angiogram; on the other hand, occult lesions may correspond to stippled hyperfluorescence, with persistent staining or leakage of dye in the overlying subretinal space in the later phases of the angiogram (fibrovascular pigment epithelial detachment), or to speckled hyperfluorescence lacking well-demarcated borders with pooling of dye in the overlying subretinal space (late-phase leakage of undetermined source). A decade later Yannuzzi et al. introduced another neovascular subtype in AMD with the description of retinal angiomatous proliferation (RAP) lesions [5].
With the decreasing number of FA exams performed in the routine practice, a need for a new classification was evident. K Bailey Freund et al. proposed in 2010 an elegant novel classification of neovascular AMD based on optical coherence tomography (OCT) images [6]. Three different neovascular lesions were described: type 1 neovascular lesions occurring beneath the RPE monolayer; type 2 neovascular lesions where the neovascular tissue has penetrated the RPE/Bruch membrane complex and is proliferating in the subretinal space above the RPE monolayer; and type 3 neovascular lesions corresponding to RAP. In addition, cases with a mixture of these features were considered mixed neovascular lesions. This OCT-based classification offers a chance to easily classify neovascular AMD according to precise tomographic signs. Thereafter, Freund et al. examined the clinical implications of such approach establishing the prognostic value of identifying each neovascular lesion subtype.
Clinical implications of the OCT-based classification of neovascular AMD
Tomographic features of neovascular subtypes
The main feature of type 1 neovascular lesions is the location bounded inferiorly by the hyperreflective remainder of Bruch membrane and superiorly by the intact hyperreflective RPE band [6]. This correlates with the first pathologic evidence reported by Grossniklaus and Gass introducing the term type 1 neovascularisation for that located below the RPE [7]. Type 1 neovascular lesions are typical of AMD given that a possible prerequisite for its development is the presence of lipid-rich material between the RPE and Bruch membrane. One of the defining characteristics of type 1 lesions is their slow and progressive growth in response to an ischaemic environment of the outer retina, thus probably preventing the development of GA overlying areas with this neovascular tissue by supplying extra nutrition to the outer retina. Subretinal fluid (SRF) is the typical exudative manifestation of type 1 neovascular lesions, mainly of low density nature, but also combined with high density fluid. Intraretinal cysts (IRC) are infrequent.
Although regression of the neovascular complex is unlikely to develop, and complete resorption of SRF is uncommon, treatments should not address these objectives, as the presence of a small amount of SRF is well tolerated in terms of visual function, and the original aim of type 1 neovascular lesions is to provide further nutrients to the outer retina. Figure 1 illustrates the multimodal imaging appearance of a typical case of type 1 neovascular lesion.
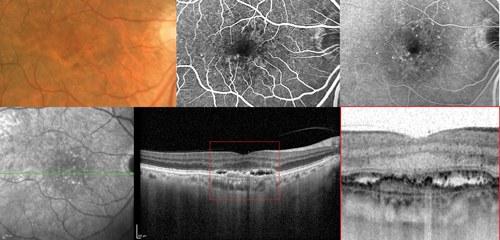
Figure 1: Type 1 neovascular lesion in neovascular age-related macular degeneration. The colour photograph shows macular pigmentary abnormalities. The fluorescein angiography images corresponding to the early and late phases evidence subtle leakage of uncertain origin corresponding to an occult neovascularisation. The optical coherence tomography scan clearly evidences type 1 neovascular lesion located beneath the retinal pigment epithelium.
The main feature of type 2 neovascular lesions is the location at the level of the subretinal space, which is reached by the neovascular complex after penetrating the hyperreflective RPE band [6]. This correlates with the first pathologic evidence reported by Grossniklaus and Gass, introducing the term type 2 neovascularisation for that located above the RPE [7]. Disorganisation of the photoreceptor ellipsoid zone is usually accompanied by IRC with variable degrees of low and high density SRF. Type 2 lesions may lead to subretinal fibrosis over the time, unless they experience a conversion to a type 1 pattern following treatment [8]. Figure 2 illustrates the multimodal imaging appearance of a typical case of type 2 neovascular lesion.
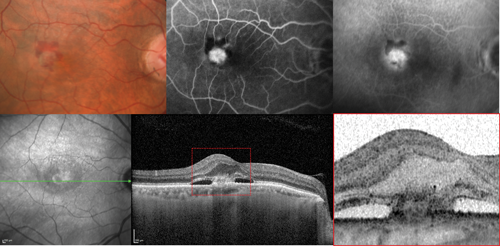
Figure 2: Type 2 neovascular lesion in neovascular age-related macular degeneration. The colour photograph shows the presence of a macular haemorrhage. The fluorescein angiography images corresponding to the early and late phases evidence classic neovascularisation. The optical coherence tomography scan clearly shows a type 2 neovascular lesion located above the retinal pigment epithelium within the subretinal space.
Finally, type 3 neovascular lesions are characterised by the heterogeneity aligned with the different stages of the disease described by Yannuzzi [5]. Initially, intraretinal hyperreflective dots are seen with surrounding small IRC usually overlying areas of damaged outer retina. As a retinal-choroidal anastomoses develops, the inner nuclear and plexiform layers adopt a funnel-shaped configuration towards the RPE hyperreflective layer. Thereafter, a detachment of the RPE may develop. All these tomographic features typically respond optimally to antiangiogenic therapy. Figure 3 illustrates the multimodal imaging appearance of a typical case of type 3 neovascular lesion.
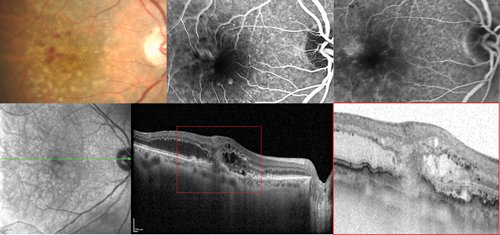
Figure 3: Type 3 neovascular lesion in neovascular age-related macular degeneration. The colour photograph shows the presence of macular microhaemorrhages and retinal oedema. The fluorescein angiography images corresponding to the early and late phases evidence retinal angiomatous proliferation. The optical coherence tomography scan clearly shows a type 3 neovascular lesion.
Prevalence of neovascular subtypes
By using FA alone the proportion of patients with occult, classic and RAP lesions showed a significant difference when compared to the use of OCT for this purpose. A total number 266 eyes in 232 patients with neovascular AMD were analysed [9]. Occult lesions as evidenced by FA accounted for 49.6% of cases, whereas type 1 lesions were estimated to represent 39.9%. Classic lesions as evidenced by FA were found in 12.0% of cases, whereas type 2 lesions by OCT were estimated to represent 9.0%. And finally, RAP lesions were identified by FA in 28.6% of cases and in 34.2% of cases by OCT. Mixed lesions represented 9.8% and 16.9% of the FA and OCT analysed images. Thus, the use of OCT enhances the capacity of clinicians to detect type 3 lesions, which might be missed when performing a FA-based approach.
Baseline prediction of visual outcome
In an observational retrospective series of 154 eyes in 138 patients with neovascular AMD managed with a treat and extend regimen (TER), those patients with type 1 neovascularisation were significantly more likely to maintain good vision compared with other neovascular subtypes [10]. It was also evidenced that vision achieved at three months following the initial intravitreal treatment was the best predictor for favourable long-term vision. The positive visual outcome was considered to be 20/60 or better. The presence of type 1 lesions evidenced a robust and statistically significant association with a positive visual outcome at two, three and four years of follow-up into a TER. On the other hand, type 3 lesions were predictive of positive visual outcome at two and three years, but the significance was lost by the fourth year. Finally, the presence of type 2 lesions was a negative predictor to achieve positive visual outcomes throughout the entire follow-up.
In an additional retrospective cohort study, Freund et al. found that the probability to improve visual acuity by three lines or more at four years of follow-up was strongly associated with neovascular subtypes [11]. A total number of 210 eyes in 185 patients were followed for a mean period of 3.5 years with a TER that required a mean number of 8.3 (±1.6) intravitreal antiangiogenic injections per year (range four to 13). Patients with type 1 neovascular lesions experienced this relevant visual gain in 42% of cases, whereas for type 2 and type 3 lesions this proportion was 12% and 27% respectively. Similarly, although lacking statistical significance, the proportion of patients with vision loss at four years of follow-up was 25%, 10%, and 45% respectively for type 1, 2 and 3 neovascular lesions. The number of injections, altogether with the anatomic classification were consistently and independently correlated with visual acuity at all time points.
Geographic atrophy development and growth
An additional study was conducted by Freund’s group to identify the initial subtypes of neovascularisation and other factors that may affect the incidence and progression of GA in newly diagnosed treatment-naive neovascular AMD eyes [12]. Areas with GA were identified using near-infrared reflectance and OCT imaging. A total number of 94 eyes in 91 patients were included. Through a mean follow-up period of 28.5 (±10.7) months, the mean number of antiangiogenic injections received was 17.4 (±9.0). The authors identified confluent areas of GA at baseline in 18.1% of cases, all of which had enlargement of their GA at last follow-up. Additionally, 37.2% of eyes that did not have apparent GA at baseline developed GA at the last follow-up.
The proportion of eyes that experienced apparent GA growth were 27.8% for cases with type 1 lesions, 57.1% for cases with type 2 lesions and 77.1% for type 3 lesions. Although controversial, the total number of injections increased the odds that an eye developed new GA or growth of existing GA; however, when the amount of apparent GA growth was analysed with multiple linear regressions in eyes with GA development, the total number of injections did not have a significant association with the quantitative amount of apparent GA growth.
Using a multiple linear regression model, which adjusts for the demographic and clinical characteristics at presentation and number of antivascular endothelial growth factor injections, and a square root transformation in order to decrease the effect of baseline GA size on GA progression, the adjusted rate of GA enlargement was 0.54 mm2 per year, 1.43 mm2 per year, and 0.80 mm2 per year for type 1, 2 and 3 neovascular lesions respectively.
What is the role of OCT-angiography today?
The potential of OCT-angiography (OCT-A) to provide further insights into the pathogenesis and activity of neovascular AMD is both evident and controversial proportionally. A myriad of findings have been described on OCT-A images with the aim to classify the activity of the neovascular lesions, the degree of maturation of the neovessels, and their possible future growth pattern [13-15]. In addition, OCT-A may constitute a tool for the early detection of recurrence of exudative manifestations in neovascular AMD [16], particularly relevant in cases of type 3 neovascular lesions [17]. Further studies are warranted in order to better assess the role of OCT-A in treatment and follow-up decisions of patients with neovascular AMD.
References
1. Rudnicka AR, Kapetanakis VV, Jarrar Z, et al. Incidence of late-stage age-related macular degeneration in American whites: systematic review and meta-analysis. Am J Ophthalmol 2015;160(1):85-93.e3.
2. Boyers LN, Karimkhani C, Hilton J, et al. Global burden of eye and vision disease as reflected in the Cochrane Database of Systematic Reviews. JAMA Ophthalmol 2015;133(1):25-31.
3. Ferris FL 3rd, Wilkinson CP, Bird A, et al; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013;120(4):844-51.
4. Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration. Guidelines for evaluation and treatment in the macular photocoagulation study. Arch Ophthalmol 1991;109(9):1242-57.
5. Yannuzzi LA, Negrão S, Iida T, et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina 2001;21(5):416-34.
6. Freund KB, Zweifel SA, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina 2010;30(9):1333-49.
7. Grossniklaus HE, Gass JD. Clinicopathologic correlations of surgically excised type 1 and type 2 submacular choroidal neovascular membranes. Am J Ophthalmol 1998;126(1):59-69.
8. Dolz-Marco R, Phasukkijwatana N, Sarraf D, Freund KB. Regression of type 2 neovascularization into a type 1 pattern after intravitreal anti-vascular endothelial growth factor therapy for neovascular age-related macular degeneration. Retina 2017;37(2):222-33.
9. Jung JJ, Chen CY, Mrejen S, et al. The incidence of neovascular subtypes in newly diagnosed neovascular age-related macular degeneration. Am J Ophthalmol 2014;158(4):769-79.e2
10. Chae B, Jung JJ, Mrejen S, et al. Baseline predictors for good versus poor visual outcomes in the treatment of neovascular age-related macular degeneration with intravitreal anti-VEGF therapy. Invest Ophthalmol Vis Sci 2015;56(9):5040-7.
11. Mrejen S, Jung JJ, Chen C, et al. Long-term visual outcomes for a treat and extend anti-vascular endothelial growth factor regimen in eyes with neovascular age-related macular degeneration. J Clin Med 2015;4(7):1380-402.
12. Xu L, Mrejen S, Jung JJ, et al. Geographic atrophy in patients receiving anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Retina 2015;35(2):176-86.
13. Coscas G, Lupidi M, Coscas F, et al. Optical coherence tomography angiography during follow-up: qualitative and quantitative analysis of mixed type I and II choroidal neovascularization after vascular endothelial growth factor trap therapy. Ophthalmic Res 2015;54(2):57-63.
14. Kuehlewein L, Bansal M, Lenis TL, et al. Optical coherence tomography angiography of type 1 Neovascularization in age-related macular degeneration. Am J Ophthalmol 2015;160(4):739-48.e2.
15. Dansingani KK, Freund KB. Optical coherence tomography angiography reveals mature, tangled vascular networks in eyes with neovascular age-related macular degeneration showing resistance to geographic atrophy. Ophthal Surg Lasers Imaging Retina 2015;46(9):907-12.
16. Lumbroso B, Rispoli M, Savastano MC, et al. Optical Coherence Tomography Angiography Study of Choroidal Neovascularization Early Response after Treatment. Dev Ophthalmol 2016;56:77-85.
17. Tan AC, Dansingani KK, Yannuzzi LA, et al. Type 3 neovascularization imaged with cross-sectional and en face optical coherence tomography angiography. Retina 2017;37(2):234-46.
Take home message
-
With the decreasing number of fluorescein angiography exams performed in routine practice, there is the need for a new classification based on optical coherence tomography images.
-
Optical coherence tomography scans of cases with neovascular age-related macular degeneration can recognise three different neovascular patterns: type 1 neovascular lesions occurring under the RPE; type 2 neovascular lesions occurring above the RPE; and type 3 neovascular lesions corresponding to retinal angiomatous proliferation.
-
The neovascular subtype in age-related macular degeneration may predict the long-term visual outcome, with type 1 lesions associated with the highest and type 2 lesions with the lowest chance for a final visual acuity of 20/60 or better.
-
The neovascular subtype in age-related macular degeneration may predict the development and growth of geographic atrophy, with type 1 lesions associated with the lowest and type 3 lesions with the highest odds.
Declaration of competing interests: None declared.
Acknowledgment
I thank Dr K Bailey Freund for providing the entire retinal physicians community his original insights into the anatomic classification of neovascular AMD, and for being a great mentor. And I acknowledge his entire research team in this field: Sarah Mrejen, Jesse J Jung, Christine Y Chen, Luna Xu, Marcela Marsiglia, Sucharita Boddu and Bora Chae.
COMMENTS ARE WELCOME