The 23rd European Society of Retina Specialists (EURETINA) Congress, held in Amsterdam, the Netherlands, provided a timely update on anti-complement therapy for geographic atrophy and emerging investigational therapies for neovascular age-related macular degeneration.
Anti-complement therapy for geographic atrophy
Geographic atrophy (GA) secondary to age-related macular degeneration (AMD) is characterised by progressive and irreversible atrophy of retinal cells and is a leading cause of severe vision loss worldwide [1].
Pegcetacoplan injection (Syfovre, Apellis Pharmaceuticals, Inc.) was approved by the US Food and Drug Administration (FDA) in February 2023 for the treatment of GA [2]. The recommended dosing regimen is once every 25-60 days. Marketing applications are currently under review with five regulatory agencies worldwide. A decision in the EU is expected in early 2024, and decisions in Canada, Australia, Switzerland and the UK are expected in the first half of 2024.
Pegcetacoplan injection binds to complement protein C3 and its activation fragment C3b with high affinity, thereby regulating the cleavage of C3 and the generation of downstream effectors of complement activation.
Avacincaptad pegol intravitreal solution (Izervay, Iveric Bio / Astellas Pharma Inc.) was approved by the US FDA in August 2023 for the treatment of GA and is currently under review by the European Medicines Agency (EMA). The recommended dosage regimen in the US label is 2mg (0.1mL of 20mg/mL solution) administered by intravitreal injection once monthly for up to 12 months [3]. Avacincaptad pegol is an RNA aptamer, a PEGylated oligonucleotide that binds to and inhibits complement protein C5.
The goal of anti-complement treatment of GA secondary to AMD is the preservation of retina cells (retinal pigment epithelium, photoreceptors and choriocapillaris) and preserving vision in the long term.

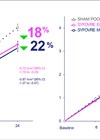
Figure 1: OPTOS symposium presentation by Prof Srinivas Sadda,
EURETINA 2023 Congress, Amsterdam, the Netherlands (courtesy of Optos).
Pegcetacoplan phase III OAKS / DERBY: 24-month results
Two-year results from phase III OAKS and DERBY trials of pegcetacoplan in patients with GA show slowing of GA lesion growth compared with sham, with increasing treatment effects over time and an acceptable safety profile [4]. At 24 months, pegcetacoplan monthly and pegcetacoplan every other month slowed GA lesion growth by 22% and 18% in OAKS, and by 19% and 16% in DERBY, respectively, compared with sham. There were no differences in key secondary visual function endpoints at 24 months. Rates of new-onset neovascular AMD (nAMD) ranged from 6-13% for pegcetacoplan and 2-4% for sham at 24 months.
Phase III GATHER2 study of avacincaptad pegol: 24-month results
Two-year results from phase III GATHER2 clinical trial met the primary objective of reducing the rate of GA growth in patients treated with monthly avacincaptad pegol compared with sham; additionally, the treatment effect with the every-other-month dosing regimen for avacincaptad pegol showed a similar reduction in the rate of GA growth versus sham [5]. Safety data were consistent with 12-month results, with no new safety signals identified. The rate of choroidal neovascularisation (CNV) development was 12% in patients treated with avacincaptad pegol and 9% in those treated with sham.
Update on dry AMD
Professor Frank G Holz (Germany) provided an update on emerging therapies for GA and possible patient selection criteria for anti-complement therapy [6]. He explained that GA is relentless and continues to progress beyond the macula. He distinguished three therapeutic scenarios: (1) slow progression of GA, (2) prevent development of GA and (3), if there if foveal involvement, improve or recover function in areas of atrophy (e.g., Pixium Vision’s PRIMA Bionic Vision System being evaluated in patients with bilateral atrophic AMD and best corrected visual acuity (BCVA) 20/320 or worse in study eye).
There is huge inter-individual variability in GA enlargement progression rates. Macula-wide photoreceptor degeneration is prognostic for future retinal pigment epithelium (RPE)-atrophy progression rates [7]. Also, there may be poor responders to anti-complement treatment that need to be identified. There is a need to monitor patients treated with anti-complement therapy regularly with optical coherence tomography (OCT) and / or fundus autofluorescence (FAF) -based lesion quantifications. In addition, there may be a need for combined anti-vascular endothelial growth factor (anti-VEGF) treatment to address incident nAMD.
Prof Holz noted that current thinking is focused on identifying patient sub-groups that would benefit the most from available anti-complement treatment, e.g., where one eye is already affected by atrophy in the fovea and with preserved foveal tissue in the fellow eye. “This lends to the concept that if we keep the fovea alive longer, we can postpone loss of foveal retinal sensitivity, which would be enormously clinically meaningful to our patients and they would be highly motivated to receive regular treatment,” observed Prof Holz.
Positive opportunities with respect to how AI-based tools might be used to help in the clinical management of patients with GA were highlighted, for example plotting of GA expansion rates on treatment over time using AI-based automated measurements and identifying other macular diseases that can mimic the features of AMD.
Managing expectations and possible patient selection criteria
In GATHER2, eligible patients had to be aged 50 years or older with non-centre-involving GA and BCVA between 20/25 and 20/320 in the study eye. In OAKS and DERBY, eligible patients had to be aged 60 years or older with BCVA ≥24 letters (approximately 20/320), and a clinical diagnosis of GA secondary to AMD and meeting GA lesion criteria, based on FAF imaging, including: total GA area must be ≥2.5 and ≤17.5 mm² (1 and 7 disk areas [DA] respectively); and if GA is multifocal, at least one focal lesion must be ≥1.25 mm² (0.5 DA).
Professor Sobha Sivaprasad (UK) considered managing patient expectations and suggestions for initiation of available anti-complement treatment for GA [8]. Treatment is unlikely to yield an appreciable change in visual function, symptoms due to scotomas may remain frustrating, and the effect of slowing GA enlargement rate is slow and likely unpredictable, noted Prof Sivaprasad. On average, GA lesions grow by ~1.5–2.0 mm² per year [9]. GA lesions have variable growth rates and distance to the fovea is a key determinant of visual prognosis. Progression is influenced by baseline lesion size and extrafoveal lesions are potential risk factors for GA progression.
Proposed considerations for potential treatment initiation of available anti-complement therapy include: meet clinical trial eligibility with respect to GA lesion criteria; identify fast progressors by monitoring growth rate over 6-12 months; and consider treating fast progressors and only eye patients with extrafoveal GA where the other eye is legally blind due to GA / nAMD. Compliance with the recommended dosing regimen as well as patient choice should also be considered. Patients will need to be carefully monitored on treatment for adverse events, including post-injection IOP spikes, anterior ischaemic optic atrophy and incident nAMD. Managing payor expectations will present challenges, particularly with payors who depend on outcome-related funding approvals.
Ultra-widefield FAF imaging for assessment of GA
Professor Srinivas Sadda (USA) reviewed diagnostic techniques for diagnosis and monitoring of GA, speaking in an Optos-sponsored symposium on emerging treatments and first-line diagnostics for GA (Figure 1). Enlargement of GA on FAF has been the primary endpoint in clinical trials. However, blue light FAF can be uncomfortable for patients and there may be concerns about repeated intense blue-light exposure in patients with atrophic macular disease.
The emergence of therapies for GA has heightened the importance of diagnostic strategies to identify and monitor GA, which need to performed at scale, noted Prof Sadda.
Geographic atrophy is well seen on ultra-widefield (UWF) FAF imaging. A multicentre study was undertaken to assess the reproducibility of GA measurements on Optos 532nm FAF imaging and compare these assessments with standard macular blue FAF imaging (36 eyes of 23 subjects with GA associated with AMD). Overall, results showed a high level of correlation of inter-grader reproducibility between the two modalities, with no significant differences in terms of grading and atrophy assessment.
Measurements from Optos UWF images have been physically validated, and measurements of GA lesions on UWF FAF images appear to be repeatable and consistent with other imaging modalities. Optos UWF FAF imaging appears to be a suitable tool for monitoring of GA in clinical practice and in clinical trials going forward, Prof Sadda observed.
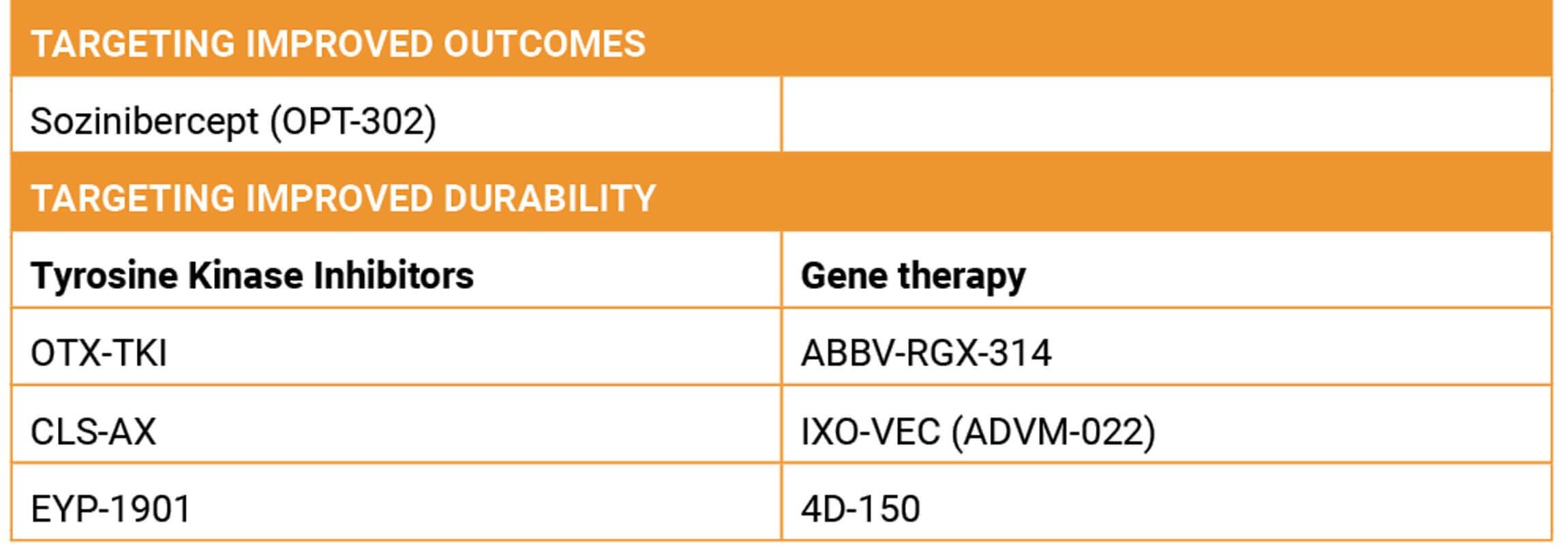
Table 1: Emerging investigational treatments for nAMD: potentially improved vision outcomes or durability.
Emerging investigational therapies for nAMD
Dr Arshad M Khanani (USA) reviewed emerging investigational therapies for nAMD, focusing on interventions that target improved outcomes or reductions in anti-VEGF injection frequency (Table 1) [10].
Intravitreal sozinibercept (OPT-302) combination therapy in nAMD
Intravitreal sozinibercept (OPT-302, Opthea) is a novel inhibitor of VEGF-C and VEGF-D. A phase II trial in treatment-naïve patients with nAMD demonstrated that monthly intravitreal 2mg OPT-302 administered in sequential combination with intravitreal ranibizumab (Lucentis, Novartis) met the pre-specified primary efficacy endpoint of a statistically superior gain in visual acuity at 24 weeks, compared with ranibizumab alone [11]. At week 24, the mean ± SD BCVA improvement in the combination arm was +14.2 ± 11.61 letters vs. +10.8 ± 11.52 letters in the ranibizumab with sham arm (P = 0.01). Improved visual acuity was also seen in patients with occult-containing lesions: mean change in BCVA at week 24 was +16.1 letters in the combination arm and +10.3 in the ranibizumab plus sham arm.
Opthea is currently conducting two global confirmatory phase III studies of sozinibercept combination therapy in treatment-naïve nAMD: ShORe (2mg OPT-302 + 0.5mg ranibizumab) and COAST (2mg OPT-302 + 2mg aflibercept [Eylea, Bayer]). Key inclusion criteria include active CNV ≥50% lesion and BCVA between 25 and 60 letters (20/63-20/320). The primary endpoint for both studies is superiority in visual acuity gains at 12 months for the combination therapy compared with standard-of-care monotherapy.
Tyrosine kinase inhibitor therapy: potent, pan-VEGF receptor inhibition
Potent, pan-VEGF receptor inhibition Axitinib is a selective tyrosine kinase inhibitor (TKI) currently being investigated via an intravitreal route (OTX-TKI, Ocular Therapeutix, Inc.) and a suprachoroidal route (CLS-AX, Clearside Biomedical, Inc.).
Ocular Therapeutix recently announced initiation of its first pivotal phase III clinical trial to evaluate OTX-TKI, axitinib intravitreal implant, for the treatment of nAMD. Designed as a superiority trial enrolling around 300 treatment-naive nAMD subjects with reasonably good VA, patients will be randomised to one aflibercept injection or one implant of OTX-TKI followed by as needed supplemental anti-VEGF treatment based on pre-specified criteria. The primary endpoint is the proportion of subjects who maintained visual acuity, defined as losing <15 ETDRS letters of BCVA from baseline at week 36 (month nine or later). Topline data from a phase I trial in nAMD showed subjects treated with a single implant of OTX-TKI were observed to have an 89% reduction in anti-VEGF treatment burden compared with subjects treated with aflibercept while maintaining visual acuity and retinal thickness through 12 months (Figure 2).
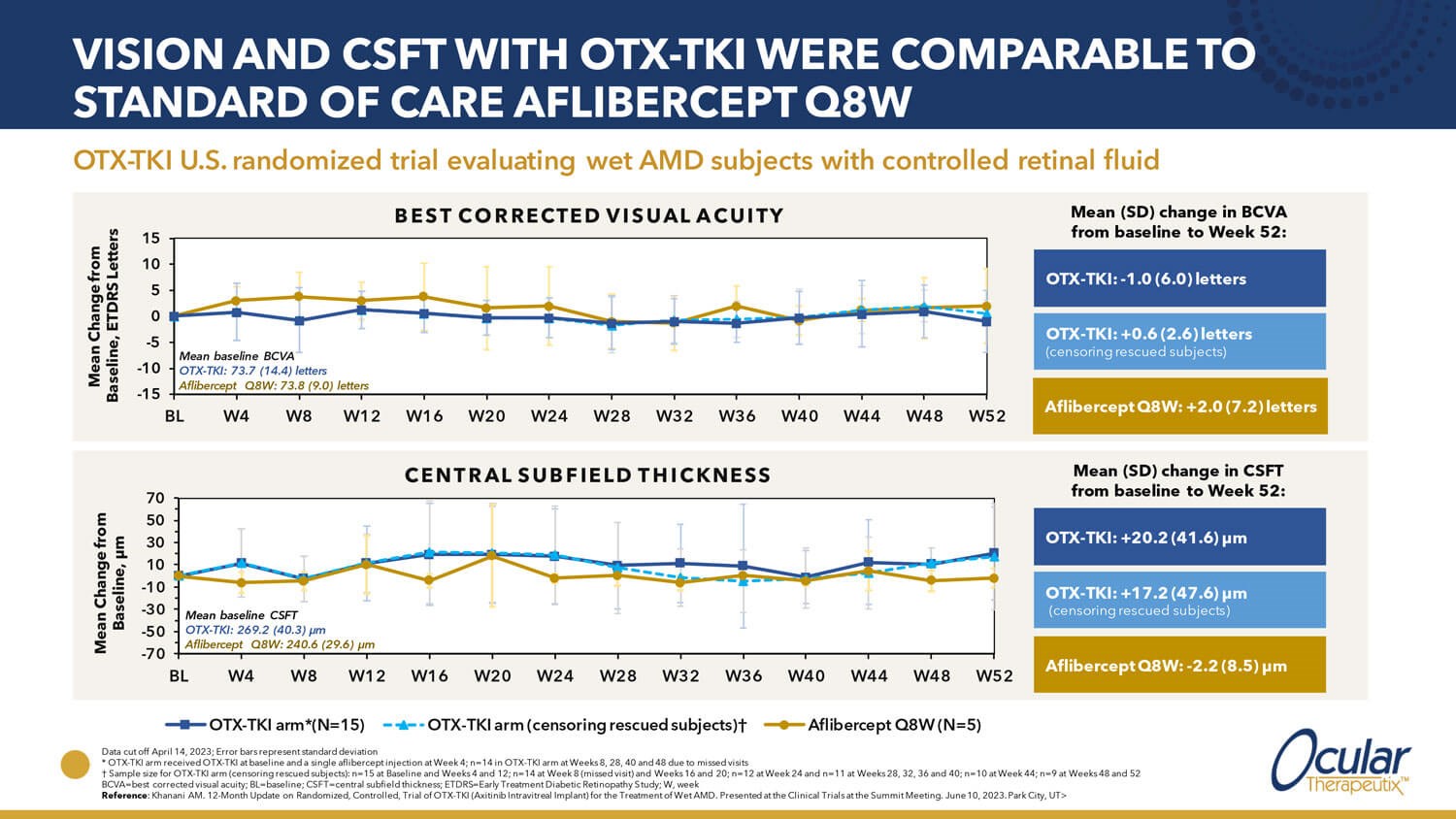
Figure 2: Phase I study evaluating OTX-TKI treatment for subjects with neovascular AMD (adapted from Ocular Therapeutix. Transformative investigational tyrosine kinase inhibitor therapy for retinal vascular diseases. Presented at: EURETINA Innovation Spotlight, Amsterdam, the Netherlands; 4 October 2023).
Phase I results showed that CLS-AX administered by suprachoroidal injection for nAMD was safe and well tolerated and exhibited early signs of durability and reduction in treatment burden. CLS-AX is being evaluated in a phase IIb clinical trial (Odyssey) for nAMD.
Sustained delivery of vorolanib for nAMD
EYP-1901 (EyePoint Pharmaceuticals, Inc.) is an investigational sustained delivery intravitreal anti-VEGF treatment currently in phase II clinical trials. EYP-1901 consists of vorolanib, a small molecule pan-VEGF receptor blocker, in a bio-erodible extended-release insert (Durasert®).
The safety and preliminary efficacy of EYP-1901 as maintenance therapy in patients with previously treated nAMD were investigated in the phase I DAVIO trial. Positive safety and efficacy data from the phase I DAVIO clinical trial of EYP-1901 in nAMD showed a positive safety profile with stable visual acuity and OCT findings. Further, the data demonstrated treatment burden reduction of 75% at six months and 73% at the 12-month visit following a single EYP-1901 injection. The median time to supplemental anti-VEGF therapy was six months. Phase II trials are fully enrolled in nAMD and non-proliferative diabetic retinopathy (DR) and a diabetic macular oedema (DMO) trial is planned for initiation in Q1 2024.
Gene therapy products
ABBV-RGX-314
ABBV-RGX-314 (AbbVie and REGENXBIO Inc.) is an investigational gene therapy for nAMD, DR and other chronic retinal conditions that cause total or partial vision loss. ABBV-RGX-314 uses the NAV® AAV8 (AAV, adeno-associated virus) vector to deliver a gene encoding a therapeutic antibody fragment to inhibit VEGF. ABBV-RGX-314 is currently being evaluated in nine ongoing clinical trials in the US and Canada. These include two pivotal trials (ATMOSPHERE and ASCENT) evaluating subretinal ABBV-RGX-314 in patients with nAMD, as well as ongoing phase II trials of subchoroidal ABBV-RGX-314 in patients with nAMD and DR (AVVIATE and ALTITUDE, respectively). ATMOSPHERE and ASCENT are expected to support global regulatory submissions with the US FDA and the EMA in late 2025 through the first half of 2026.
In July 2023, updated interim data from a phase II pharmacodynamic study was presented at the American Society of Retina Specialists annual meeting, demonstrating that subretinal delivery of ABBV-RGX-314 for nAMD was well tolerated at the same dose levels being used in the two pivotal trials [12]. Initial data in the low dose cohort through six months exhibited expected protein levels along with stable to improved BCVA and central retinal thickness, as well as meaningful reductions in anti-VEGF burden, with most subjects (11/15, 73%) remaining injection-free. Interim results from AAVIATE and ALTITUDE trials demonstrated that ABBV-RGX-314 suprachoroidal delivery administered to patients in cohorts at dose level three (1.0x1012 genome copies per eye) with short-course (seven-week) prophylactic topical steroid eye drops (n=39) resulted in zero reported cases of intraocular inflammation. Time of post-administration follow up ranged from six weeks to six months.
Ixo-vec
Ixoberogene soroparvovec (Ixo-vec) (Adverum Biotechnologies, Inc.), formerly referred to as ADVM-022, is a single, in-office intravitreal injection gene therapy product designed to deliver long-term durable therapeutic levels of aflibercept. In the phase I OPTIC study in subjects with nAMD who have demonstrated responsiveness to anti-VEGF treatment, Ixo-vec has been generally well tolerated, with the most common adverse events being dose-dependent AAV associated ocular inflammation that has been responsive to topical corticosteroid therapy. Best-corrected visual acuity and contrast sensitivity testing (CST) were maintained or improved through two years with both trial doses. A single Ixo-vec intravitreal injection resulted in sustained therapeutic aflibercept through at least three years. Mean annualised anti-VEGF injections were reduced by 80-98%, and 53% of participants who received 2x10^11 vg / eye did not receive any supplemental injection in two years.
The ongoing phase II LUNA study is evaluating 2x10^11 vg / eye and a lower 6x10^10 vg / eye dose with enhanced prophylactic corticosteroid regimens in patients with nAMD. Primary endpoints will focus on mean change in BCVA and CST from baseline to one year, and incidence and severity of adverse events.
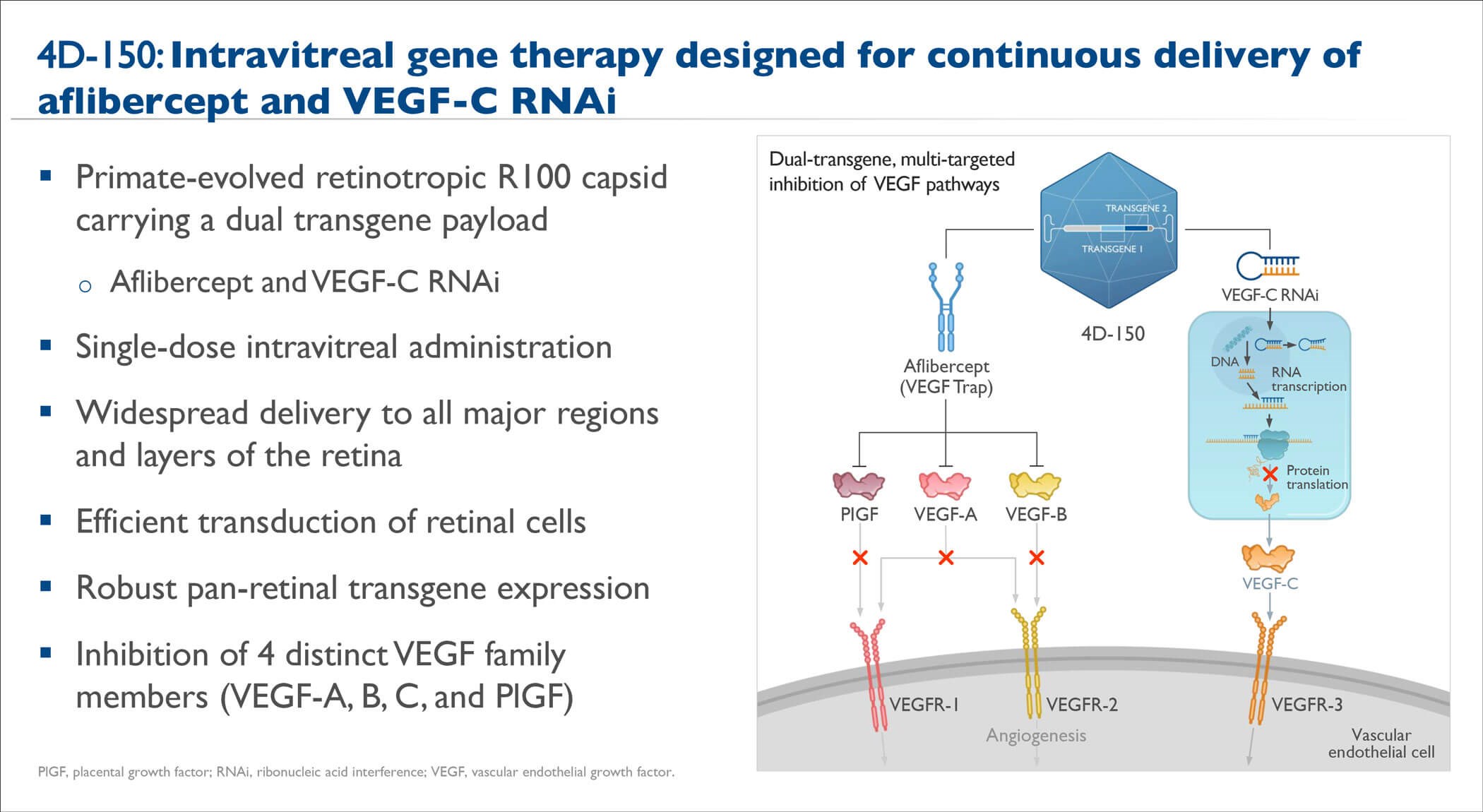
Figure 3: 4D-150 Overview (adapted from Kay CN et al. ASRS annual meeting 2023).
4D-150
4D-150 (4D Molecular Therapeutics) is an investigational genetic medicine using the intravitreal R100 vector for the treatment of nAMD and DMO (Figure 3). 4D-150 is in the randomised phase II stage of the phase I /II PRISM study for adults with nAMD and in the phase II SPECTRA study for adults with DMO.
Interim results from the PRISM trial showed that intravitreal administration of 4D-150 to adults with nAMD (clinical response to anti-VEGF therapy in prior 12 months with BCVA ≥25 and ≤78 ETDRS letters) was safe and generally well tolerated at doses ranging from 6x109 to 3x1010 vg / eye through week 36 [13]. There were no dose-limiting toxicities and no 4D-150–related serious adverse events; 14/15 participants experienced no intraocular inflammation; and there was no hypotony. Durable clinical activity was demonstrated across all 4D-150 dose cohorts through week 36, with up to 81% reduction in mean annualised anti-VEGF injection rate and stable BCVA and CST. Enrolment in a phase II dose expansion study in adults undergoing active anti-VEGF treatment who have demonstrated a clinical response (n=50) has been completed: 4D-150 (1x1010 vg / eye or 3x1010 vg / eye) vs. aflibercept 2mg.
Novel one-time gene therapy treatment could potentially reduce the treatment burden of currently available effective therapies while maintaining vision with a favourable benefit:risk profile.
TAKE HOME MESSAGE
-
Current thinking is focused on identifying patient sub-groups that would benefit the most from available anti-complement treatment for GA.
-
The emergence of therapies for GA has heightened the importance of diagnostic strategies to identify and monitor GA at scale.
-
Patients treated with anti-complement therapy will need to be monitored regularly with OCT and / or FAF-based lesion quantifications; in addition, there may be a need for combined anti-VEGF treatment to address incident nAMD.
-
Clinical trials evaluating emerging investigational therapies for nAMD are focused on demonstrating better outcomes or reductions in intravitreal injection burden compared with available effective therapies.
References
1. Fleckenstein M, Mitchell P, Freund KB, et al. The progression of geographic atrophy secondary to age-related macular degeneration. Ophthalmology 2018;125(3):369-90.
2. SYFOVRE™ (pegcetacoplan injection), for intravitreal use; US highlights of prescribing information (2023). U.S. Food and Drug Administration.
https://www.accessdata.fda.gov/
drugsatfda_docs/label/
2023/217171s000lbl.pdf
3. IZERVAY™ (avacincaptad pegol intravitreal solution); US highlights of prescribing information (2023). U.S. Food and Drug Administration.
https://www.accessdata.fda.gov/
drugsatfda_docs/label/
2023/217225s000lbl.pdf
4. Heier JS, Lad EM, Holz FG, et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase III trials. Lancet 2023;402(10411):1434-48.
5. Iveric Bio Announces Positive 24-Month Topline Results from Phase 3 Study of IZERVAY™ (avacincaptad pegol intravitreal solution) for Geographic Atrophy (2023). IVERIC BIO.
https://ivericbio.com/iveric-bio-announces
-positive-24-month-topline-results-from-phase
-3-study-of-izervay-avacincaptad-pegol-intravitreal
-solution-for-geographic-atrophy/
6. Holz FG. Update dry AMD therapies and patient selection criteria. 23rd European Society of Retina Specialists Congress: 5-8 October 2023; Amsterdam, the Netherlands.
7. Pfau M, von der Emde L, de Sisternes L, et al. Progression of photoreceptor degeneration in geographic atrophy secondary to age-related macular degeneration. JAMA Ophthalmol 2020;138(10):1026-34.
8. Sivaprasad S. Clinical expectations with treatment of GA Rx in 2023. 23rd European Society of Retina Specialists Congress: 5-8 October 2023; Amsterdam, the Netherlands.
9. Lindblad AS, Lloyd PC, Clemons TE, et al; Age-Related Eye Disease Study Research Group. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol 2009;127(9):1168-74.
10. Khanani AM. Emerging therapies for exudative AMD. 23rd European Society of Retina Specialists Congress: 5-8 October 2023; Amsterdam, the Netherlands.
11. Jackson TL, Slakter J, Buyse M, et al; Opthea Study Group Investigators. A randomized controlled trial of OPT-302, a VEGF-C/D inhibitor for neovascular age-related macular degeneration. Ophthalmology 2023;130(6):588-97.
12. Abbey AM. Subretinal delivery of ABBV-RGX-314 for neovascular AMD: a phase II pharmacodynamic study. American Society of Retina Specialists (ASRS) Annual Meeting: 28 July-1 August 2023; Seattle, WA, US.
13. Kay CN, Khanani AM, Hershberger VS, et al. Interim results of the phase I/2 PRISM trial evaluating 4D-150, a dual-transgene intravitreal genetic medicine for neovascular (wet) age-related macular degeneration. American Society of Retina Specialists (ASRS) Annual Meeting: 28 July-1 August 2023; Seattle, WA, US.
[all links last accessed October 2023]
Declaration of competing interests: The author has provided consultancy services to Bayer AG, DORC International B.V., Johnson & Johnson Vision Care, Inc., Roche Products Ltd and Thea Pharmaceuticals Ltd.
COMMENTS ARE WELCOME