High myopia is defined as myopic refraction of greater than -6 dioptres with an axial length greater than 26.5mm, while pathological myopia is myopic refraction with posterior pole degeneration [1]. These degenerative changes can affect a young population and in unrecognised cases, can cause irreversible vision loss. Available investigations have given us a chance to diagnose posterior pole changes early, and these aid our clinical assessment. This article aims to review the various posterior segment changes seen in patients with pathological myopia.
Introduction
The World Health Organization (WHO) defines myopia as a refractive error in which rays of light entering the eye parallel to the optic axis are brought to focus in front of the retina when accommodation is relaxed [1]. The term ‘high myopia’ is defined as a myopic refractive error of greater than -6 dioptres and axial length greater than 26.5mm [3]. Pathological myopia was traditionally defined as a myopic refraction with posterior pole myopic degenerative changes.
The degenerative changes in the posterior pole, though very common in people with high myopia (50–70%), also occur in 1–19% percent of patients with mild to moderate myopia [2]. The pathogenesis for pathological myopia is axial elongation with associated abnormal collagen proteins which leads to the degenerative changes in the retina, Bruch’s membrane, choroid and sclera. Choroidal thinning was hypothesised to be an important factor in visual impairment in patients with pathological myopia [4].
Clinical features of pathological myopia
1. Tessellated posterior pole
Tessellated fundus is common in high myopes. This tigroid appearance is because of the prominently visible choroidal vessels following depigmentation and retinal pigment epithelium (RPE) atrophy (Figure 2).There is an inverse relationship between the tessellated appearance and the axial length which means patients with axial length of greater than 26mm have less chance of having a tessellated appearance, as they would have advanced fundus changes like degeneration and atrophy due to high axial length [9].
2. Peripapillary atrophy
Peripapillary atrophy is the chorio-retinal atrophy due to thinning and disruption of RPE surrounding the disc [10]. This being a nonspecific sign, it can occur in glaucoma, ocular hypertension, and high myopia [11].
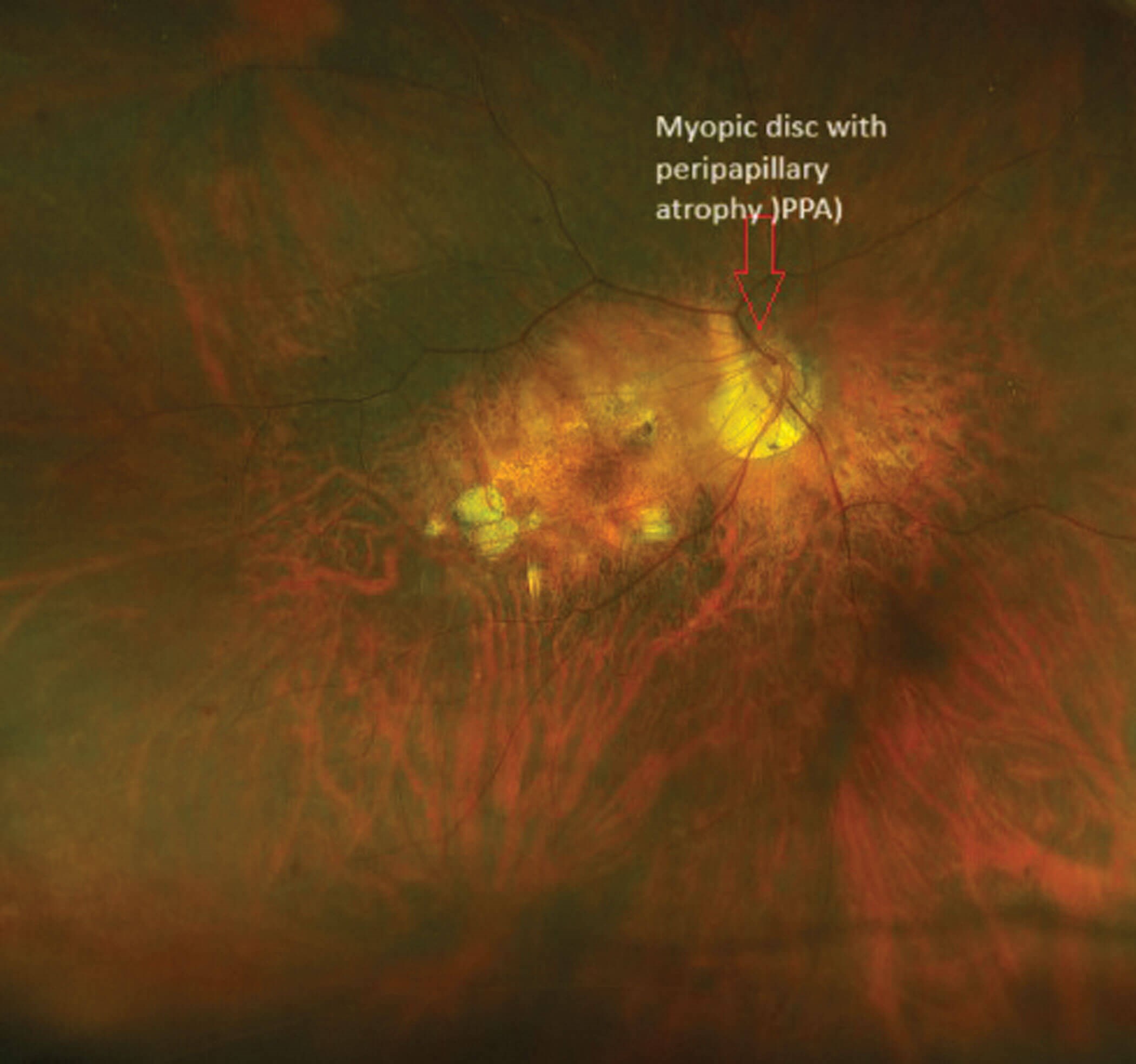
Figure 1: Right eye with a myopic disc with peripapillary atrophy and pigmentary changes in the macula.
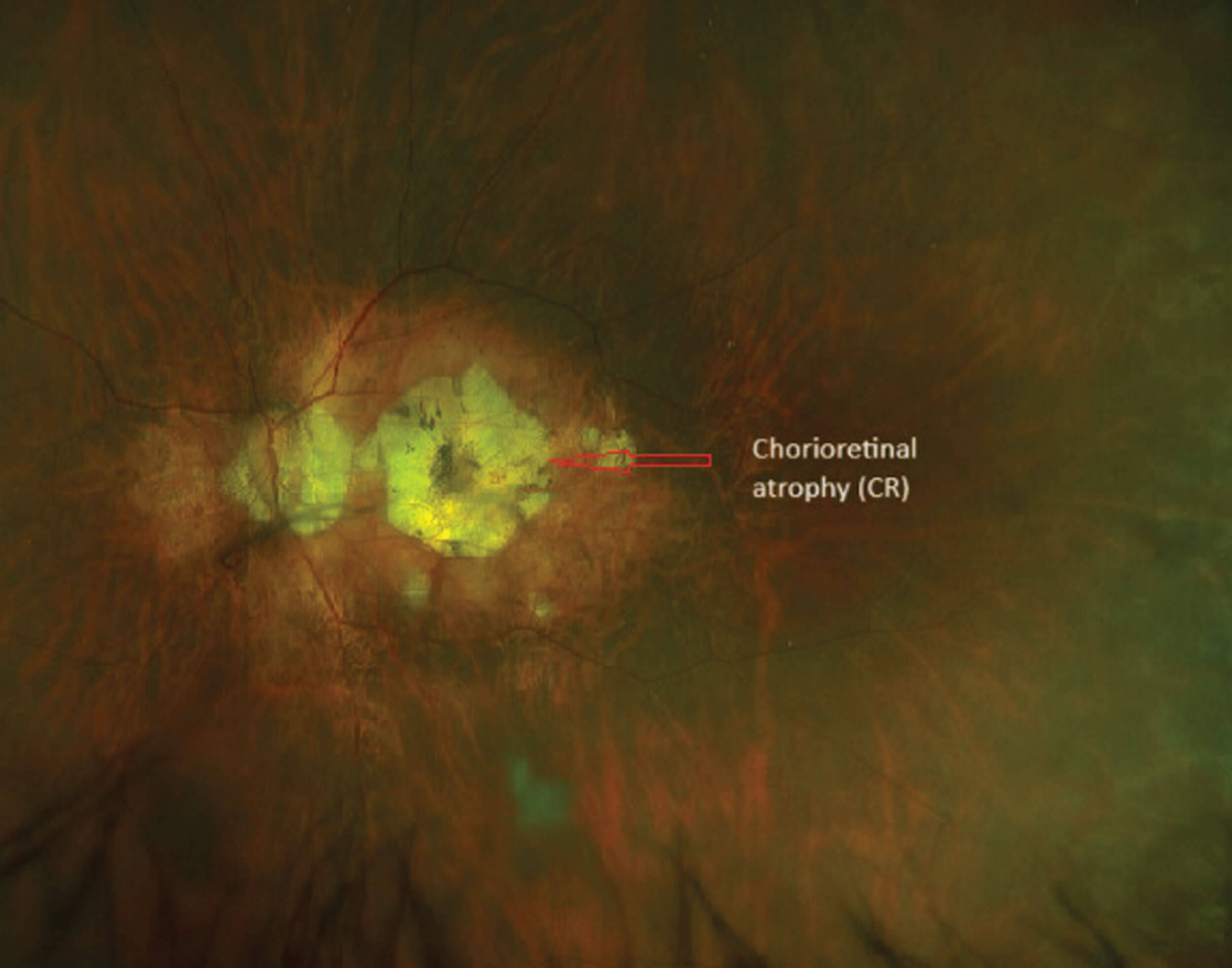
Figure 2: Left eye with myopic disc with peripapillary atrophy and complete atrophy of the macula.
3. Myopic disc
Myopic patients can have structural changes in the optic nerve due to the elongation of the globe and disproportionate stretching of the sclera, choroid and retina. The disc can be tilted due to the insertion of the optic nerve at an angle (Figure 1). Patients can also have a large disc with large rim area and parapapillary atrophy [22].
4. Chorio-retinal atrophy
The increase in the axial length in high myopia can lead to progressive thinning of the choroid and its vasculature. The photoreceptors receive their nutrition from the choroid. The thinning of choroid and its vasculature leads to loss of photoreceptors and RPE loss. Chorio-retinal atrophy can occur in the peripheral retina or involve the macular region. Chorio-retinal atrophy involving the macular region can be patchy or diffuse with both types being a predisposing factor for formation of choroidal neovascular membrane (CNV) [12].
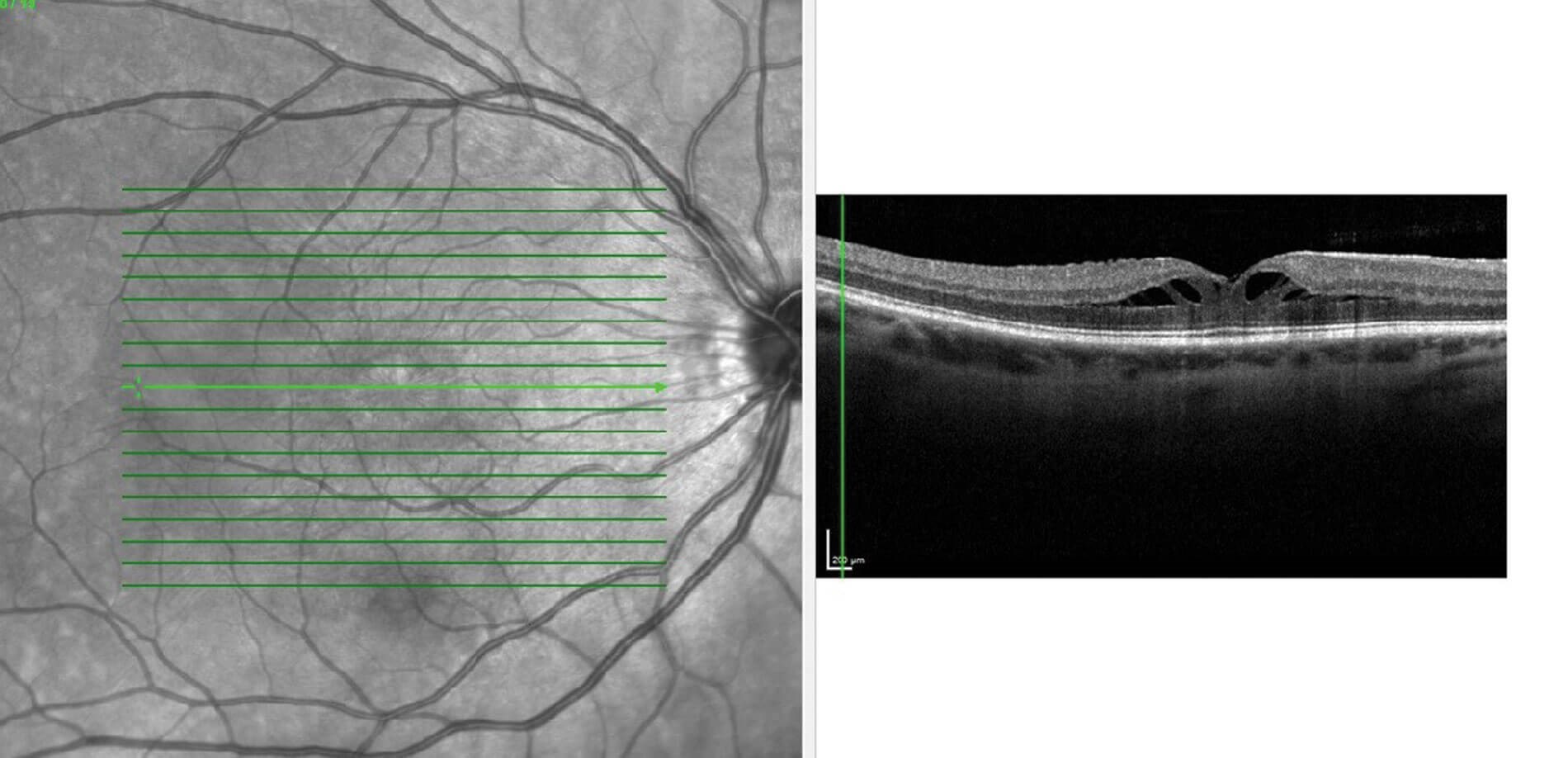
Figure 3: OCT macula of the right eye with foveal schisis.
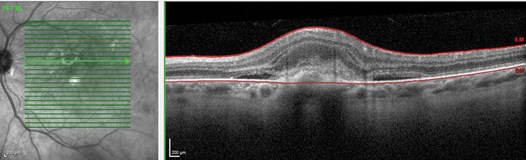
Figure 4: OCT macula showing a myopic CNV.
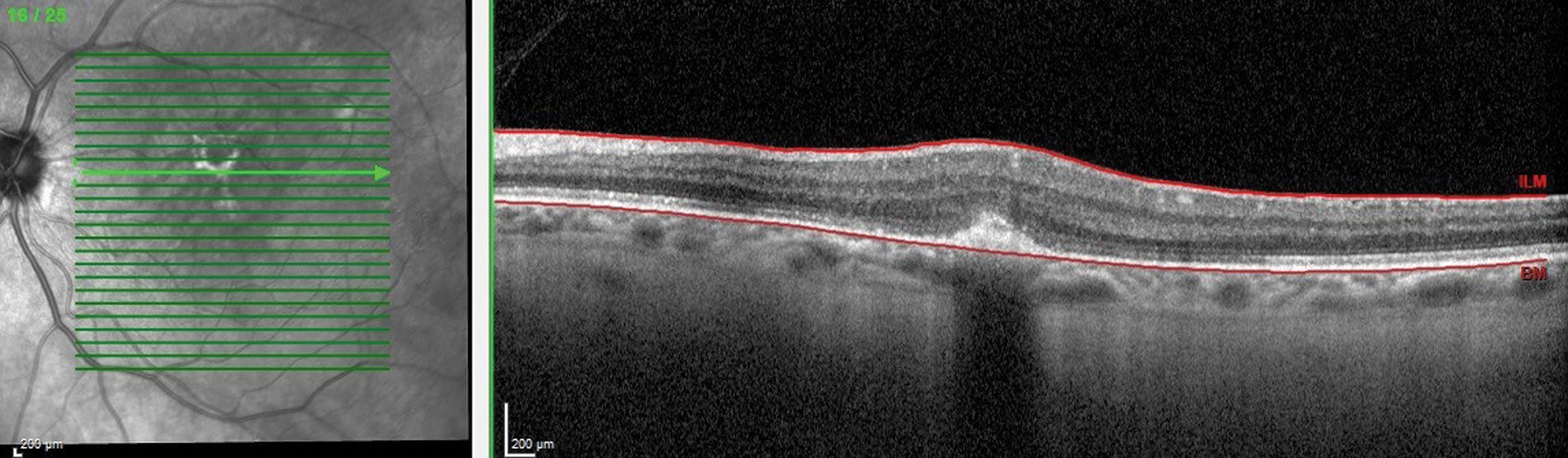
Figure 5: OCT macula of the same patient after receiving the first dose of anti-VEGF.
Myopic CNV
One of the commonest causes of vision impairment in patients with pathological myopia is choroidal neovascular membranes. The fundus examination reveals a greyish membrane in the macular region with or without associated haemorrhage. Choroidal neovascular membrane arises from the choroid and initially lies within the Bruch’s membrane, gradually piercing the RPE and staying under the neurosensory retina [13]. There are three different types of CNV:
- Type I (also known as the ‘occult’ type) in which the neovascular membrane lies between the RPE and Bruch’s membrane.
- Type II (also known as the ‘classical’ type) in which the neovascular membrane lies above the RPE.
- Type III (also known as the known as ‘retinal angiomatous proliferation’) in which new vessels proliferate within the neurosensory layer of the retina.
In myopia, the neovascular membrane predominantly is of type II [3]. In optical coherence tomography (OCT), the neovascular membrane appears as a hyperreflective lesion in the subretinal region (Figure 4). The most preferred treatment option for myopic CNV is anti-VEGF (Figure 5 shows the organised hyperreflective material after anti-VEGF treatment). Fundus fluorescein angiography is considered the gold-standard in investigating and assessing the activity of CNV [14]. Recently, OCT angiography (OCT-A) serves as a useful non-invasive tool to diagnose neovascularisation. In OCT-A, the neovascular membrane can appear as a lazy wheel-shaped lesion, tiny capillary branching, or as an anastomotic loop [14].
The treatment options available for myopic CNV initially, before the use of anti-VEGF therapy, was laser photocoagulation and photodynamic therapy (PDT) with verteporfin. Ranibizumab was the first anti-VEGF to be approved by the North American Food and Drug Administration (FDA) in 2017. Many trials were conducted to analyse the efficacy of ranibizumab in myopic CNV.
Of note is the RADIANCE study (double blinded phase III clinical trial), which was done to compare the efficacy of ranibizumab and PDT with verteporfin in patients with CNV secondary to pathological myopia (15). The study concluded that patients on ranibizumab had superior best corrected visual acuity gains compared to patients to patients on PDT.
BRILLIANCE was a similar study conducted on Asian patients with myopic CNV. In this study, patients were randomised into three groups: ranibizumab guided by visual acuity; ranibizumab guided by disease activity; and PDT followed by ranibizumab / PDT / both [16]. This study concluded that patients on ranibizumab had superior efficacy compared to verteporfin PDT at month three, and the beneficial treatment effects persisted at month 12 [16].
The MYRROR study was conducted to determine the influence of baseline myopic macular degeneration (MMD) severity on outcomes with intravitreal aflibercept – the visual acuity gains, morphological outcomes, and dosing frequency were not affected by baseline MMD severity in patients treated with intravitreal aflibercept (17). Untreated, myopic CNV can cause irreversible vision loss of central vision due to scarring.
5. Posterior staphyloma
Posterior staphyloma, also known as ‘Scarpa’s staphyloma’, is an outpouching (or bulging) of the posterior pole due to the degenerative changes. The radius of curvature of the posterior staphyloma is smaller than the adjacent wall’s curvature [2]. Scleral and choroidal thinning secondary to increasing axial length have been proposed to be the reason for posterior staphyloma. On dilated fundus examination, staphyloma will appear as a depression with vessels dipping at the margin.
6. Myopic retinal detachment
Rhegmatogenous retinal detachment (RRD) is a sight-threatening emergency, which is more common in high myopes than emmetropes because of the presence of retinal hole and peripheral lattice degeneration. The predisposing factor for the RRD is the presence of a retinal tear and degenerated vitreous and premature vitreous detachment. The retinal tear with firm vitreous traction serves as a site through which the liquefied vitreous can seep through to cause the retinal detachment [22,23]. It is important to examine the other eye for presence of retinal holes / tears in the periphery as the incidence of retinal detachment in the fellow eye of a high myope is 16.7% [8]. Cataract surgery increases the risk of retinal detachment in myopic eyes [24] and the risk was highest in the first six months [25].
7. Myopic traction maculopathy
Myopic traction maculopathy (MTM), also known as foveal schisis was first described by Takano and Kishi in 1999, when they noticed a break in the foveal region in patients with posterior staphyloma [20]. The pathogenesis for development of foveal schisis was due to the abnormal elongation of the sclera in the presence of different stretching capacity of choroid and the retina. This causes parallel and perpendicular stretching force in the retina leading to thickening of inner retinal layers and thinning of outer retinal layers (Figure 3) Other contributing factors for fovealschisis could be inter-limiting membrane rigidity, vitreomacular traction, and epiretinal membrane [20]. Raizada, et al. described an OCT-based classification of MTM:
- Inner schisis or inner outer schisis
- Outer schisis
- Schisis detachment
- Detachment
8. Lacquer cracks
A lacquer crack is a break in the Bruch’s membrane which appear as linear, yellowish lesions when examined by ophthalmoscope. Though the exact mechanism is not completely understood, it is hypothesised that the elongation of the globe can cause a break in the Bruch’s membrane and choriocapillaris. In fundus angiography they appear as a window defect. At times, the break can be associated with subretinal haemorrhage which can have an appearance of a coin [18]. Fundus angiography can help to differentiate and rule out haemorrhage due to choroidal neovascularisation. Lacquer cracks can also be associated with choroidal neovascularisation which occur at the margin or can be in an adjacent region [3].
"Ranibizumab had superior efficacy compared to verteporfin PDT at month three, and the beneficial treatment effects persisted at month 12" [16]
9. Fuchs spot
Fuchs spot, commonly referred to as Forster Fuchs spot, named after Forster who first described the subretinal neovascularisation in 1862 and Ernst Fuchs who described the pigmented lesion in 1901 [3]. The frequency of occurrence of Fuchs spot in myopic patients range from 5–10% [19]. Fuchs spots are pigmented lesions due to RPE proliferation at the site of regressed choroidal neovascularisation.
Conclusion
High myopia is defined as myopic refraction of greater than -6D with an axial length greater than 26.5mm while pathological myopia is myopic refraction with posterior pole degenerative changes. Due to the axial elongation, degenerative changes occur in the retina, Bruch’s membrane, choroid and sclera. This article emphasises on the important optic disc and retinal findings in a person with pathological myopia.
TAKE HOME MESSAGES
-
High myopia is defined as a myopic refractive error refractive of greater than -6 dioptres and axial length greater than 26.5mm.
-
Pathological myopia is defined as a myopic refraction with posterior pole myopic degenerative changes.
-
Untreated, myopic CNV can cause irreversible vision loss of central vision due to scarring.
-
High myopia is more prone to retinal detachment because of the retinal holes and peripheral retinal degeneration.
References
1. Flitcroft DI, He M, Jonas JB, et al. IMI – Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Invest Ophthalmol Vis Sci 2019;60(3):M20–30.
2. Ohno-Matsui K, Wu PC, Yamashiro K, et al. IMI Pathologic Myopia. Invest Ophthalmol Vis Sci 2021;62(5):5.
3. Kumar A, Chawla R, Kumawat D, Pillay G. Insight into high myopia and the macula. Indian J Ophthalmol 2017;65:85–91.
4. Wang Y, Chen S, Lin J, et al. Vascular Changes of the Choroid and Their Correlations With Visual Acuity in Pathological Myopia. Invest Ophthalmol Vis Sci 2022;63(12):20.
5. Ohno-Matsui K, Ikuno Y, Lai TYY, Gemmy Cheung CM. Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog Retin Eye Res 2018;63:92–106.
6. Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International Photographic classification and grading system for myopic maculopathy. Am J Ophthalmol 2015;159(5):877–83.
7. Parolini B, Palmieri M, Finzi A, et al. The new Myopic Traction Maculopathy Staging System. Eur J Ophthalmol 2021;31(3):1299–312.
8. Orihara T, Hirota K, Yokota R, et al. [Clinical Characteristics of Rhegmatogenous Retinal Detachment in Highly Myopic and Phakic Eyes]. Nippon Ganka Gakkai Zasshi 2016;120(5):382–9.
9. Tokoro T. Atlas of Posterior Fundus Changes in Pathologic Myopia. Japan, Tokyo; Springer Tokyo; 1998.
10. Manjunath V, Shah H, Fujimoto JG, Duker JS. Analysis of peripapillary atrophy using spectral domain optical coherence tomography. Ophthalmology 2011;118(3):531–6.
11. Uchida H, Ugurlu S, Caprioli J. Increasing peripapillary atrophy is associated with progressive glaucoma. Ophthalmology 1998;105(8):1541–5.
12. Ohno-Matsui K, Yoshida T, Futagami S, et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol 2003;87(5):570–3.
13. Bhatt NS, Diamond JG, Jalali S, Das T. Choroidal neovascular membrane. Indian J Ophthalmol 1998;46(2):67–80.
14. Li S, Sun L, Zhao X, et al. Assessing the activity of myopic choroidal neovascularization: Comparison Between Optical Coherence Tomography Angiography and Dye Angiography. Retina 2020;40(9):1757–64.
15. Wolf S, Balciuniene VJ, Laganovska G, et al. RADIANCE Study Group. RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology 2014;121(3):682–92.
16. Chen Y, Sharma T, Li X, et al. Ranibizumab versus verteporfin photodynamic therapy in asian patients with myopic choroidal neovascularization: BRILLIANCE, a 12-month, randomized, double-maksed study. Retina 2019;39(10):1985–94.
17. Cheung CMG, Ohno-Matsui K, Wong TY, et al. MYRROR Study Investigators. Influence of myopic macular degeneration severity on treatment outcomes with intravitreal aflibercept in the MYRROR study. Acta Ophthalmol 2019;97(5):e729–35.
18. Chang KJ, Cheng CK, Peng CH. Clinical characteristics and visual outcome of macular hemorrhage in pathological myopia with or without choroidal neovascularization. Taiwan J Ophthalmol 2016;6(3):136–40.
19. Fledelius HC, Alsbirk PH, Goldschmidt E (Eds.). Third International Conference on Myopia Copenhagen, August 24–27, 1980. Dordrecht, Netherlands; Springer Dordrecht; 1981.
20. Raizada K, Sahu J. Myopic Foveoschisis. StatPearls [Internet]. Treasure Island (FL), USA; StatPearls Publishing; 2022. PMID: 33085274.
21. Cheng D, Ruan K, Wu M, et al. Characteristics of the Optic Nerve Head in Myopic Eyes Using Swept-Source Optical Coherence Tomography. Invest Ophthalmol Vis Sci 2022;63(6):20.
22. Williams K, Hammond C. High myopia and its risks. Community Eye Health 2019;32(105):5–6.
23. van Leeuwen R, Haarman AEG, van de Put MAJ, et al; Association of Rhegmatogenous Retinal Detachment Incidence With Myopia Prevalence in the Netherlands. JAMA Ophthalmol 2021;139(1):85–92.
24. Chou SC, Yang CH, Lee CH, et al. Characteristics of primary rhegmatogenous retinal detachment in Taiwan. Eye (Lond) 2007;21(8):1056–61.
25. El-Abiary M, Shams F, Goudie C, Yorston D. The Scottish RD survey 10 years on: the increasing incidence of retinal detachments. Eye (Lond) 2022;37(7):1320–4.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME