Introduction
Formerly known as visual impairment and intracranial pressure syndrome (VIIP), space-related neuro-ocular syndrome (SANS) is defined by a collection of ophthalmic and neurological findings in astronauts after long-term spaceflight [1].
Changes in the eyeball, such as hyperopic shift, during short-term spaceflight ( less than two weeks, have previously been observed by NASA. Other constellations of SANS-defining signs include choroidal & retinal folds, cotton wool spots, optic disc oedema, optic nerve sheath distension, and globe flattening [2].
Symptoms include visual scotomas, deterioration of distance vision, headaches and decreased near-visual acuity. Self-reported SANS-related symptoms correlated with spaceflight time in a dose-dependent manner with 23% claiming disturbances in near-sightedness after short-duration space flight (SDSF) and up to 47% claiming similar symptoms after long-duration space flight (LDSF) onboard the International Space Station (ISS) [3].
Pathogenesis
Although the exact mechanism and pathophysiology of these eye changes in astronauts are not yet clear, some studies have found that astronauts experience raised intracranial pressure (ICP), biochemical and neurological changes, and lymphatic and venous system circulation disorders after entering a microgravity environment. It is thought that these factors play a major role in the development of the condition.
Two theories have been described in the literature, with the first one known as the cephalad fluid shift theory. Increased intracranial volume and pressure may be caused by fluid shifts within the brain and elevated cerebrospinal fluid (CSF) pressure around the brain may subsequently occur within the optic nerve (ON) sheaths thereby causing disc oedema as well as globe flattening. Few studies have shown that cerebral autoregulation is maintained during spaceflight, however venous stasis in both the femoral and jugular veins has been observed during spaceflight. As a result, this mimics a terrestrial idiopathic intracranial hypertension (IIH)-like picture as it suggests venous congestion which might impact CSF outflow. This congestion may also inhibit vortex vein drainage and cause choroidal thickening that could add to axial shortening and the observed hyperopic shift. The ‘IIH-like’ theory is arguable due to absence of classic symptoms of terrestrial IIH such as headache, transient visual changes, tinnitus, nausea, vomiting and the lack of recordable inflight CSF opening pressures [4].
Another proposed hypothesis is that microgravity-induced changes in the physiology of CSF and minor variations in physiology of flow and drainage within the optic nerve sheath itself causes SANS. It is suggested that a one-way valve-like system is created by the ON sheath, causing CSF compartmentalisation within the optic nerve sheath without elevating the CSF around the brain [2].
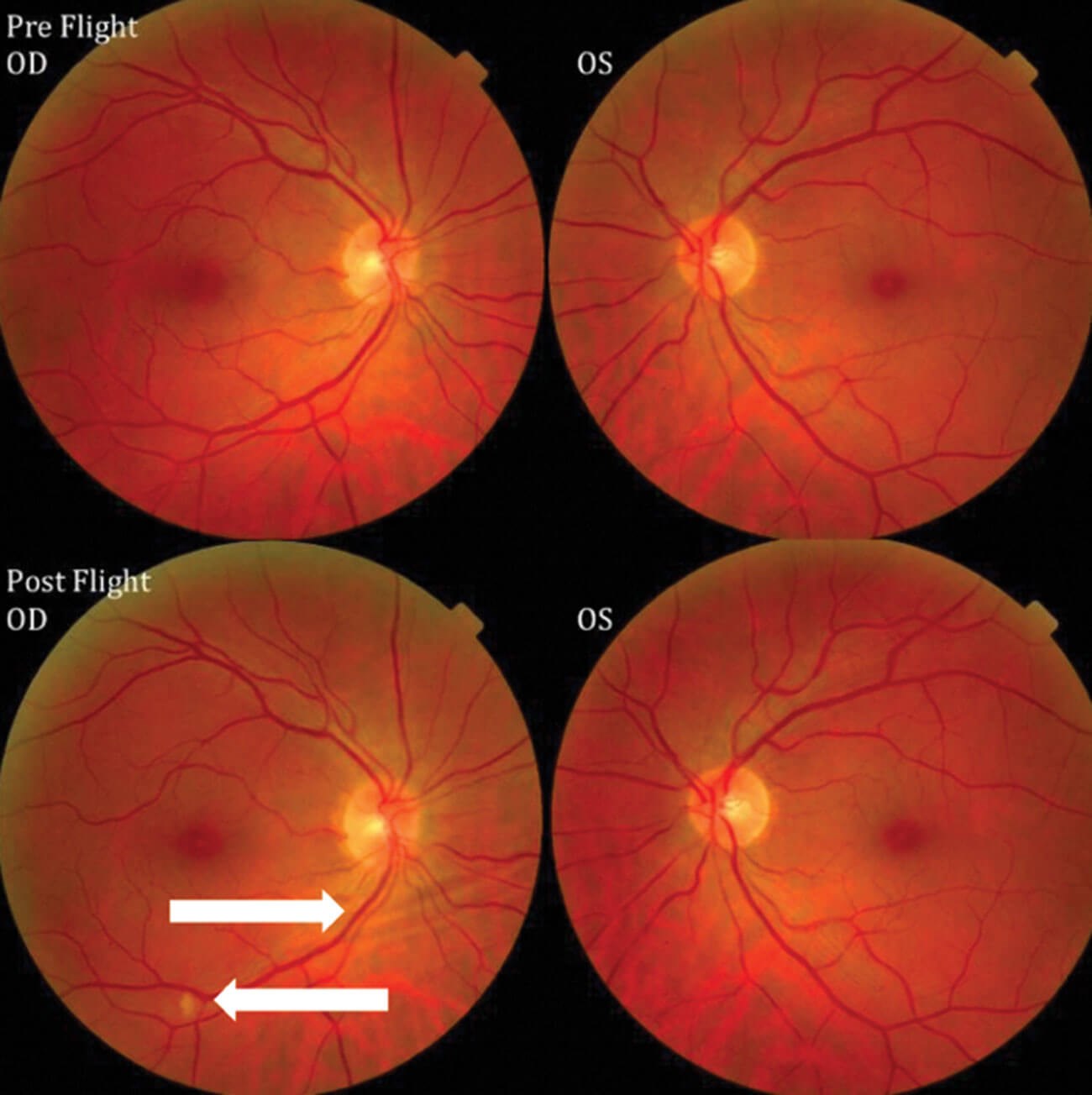
Figure 1: Fundus examination of first case of vision changes from long-duration spaceflight. Fundus examination revealed choroidal folds in inferior to the optic disc (right-pointing arrow) and a single cotton wool spot (left-pointing arrow) in the inferior arcade of the right eye. Source: NASA Risk of Spaceflight Associated Neuro-ocular Syndrome Evidence Report.
Diagnosis
Visual acuity findings show increased hyperopic sphere. Normal to near normal visual acuity is noted on cycloplegic and manifest refraction. Fundus examination may show cotton wool spots, disc oedema and choroidal folds. The evidence demonstrates that SANS commonly presents as asymptomatic bilateral non-symmetric optic disc oedema [4].
To diagnose SANS, astronauts have undergone several tests to include pre- and post-flight magnetic resonance imaging (MRI), pre-, intra-, and post-flight optical coherence tomography (OCT), post-flight lumbar puncture and orbital ultrasound. Imaging findings from these procedures have the potential to demonstrate structural abnormalities which may correlate to the clinical findings seen in SANS such as optic disc oedema, dilation of ON sheath and globe flattening. The OCT scans can reveal changes such as tortuosity and thickening of the optic nerve head [4].
In the astronauts exposed to microgravity environments, posterior globe flattening, and pituitary dome concavity was also noted [5]. Fluid shifts within the brain have been shown on post-flight MRI. Limitations to inflight diagnostic procedures are caused by equipment portability and technical factors. Orbital ultrasound, fundoscopic examinations and OCT scans are used onboard the ISS, all of which have a role in detecting globe flattening, optic disc oedema, chorioretinal folds and cotton wool spots. More recently, with the introduction of the OCT angiography (OCTA) on the ISS, changes in choroidal blood flow may better our understanding of the cephalad fluid shift theory and SANS [6].
Lumbar punctures performed on astronauts post flight have been shown to cause raised CSF opening pressures, in some cases as high as 28.5 cmH2O two months after landing. Several findings demonstrate similarities to terrestrial IIH; however, ocular findings in SANS has a tendency to present asymmetrically in contrast to IIH [4].
Evidence from NASA has shown a link between enzymatic deficiencies in folate dependent 1-carbon pathway and cyanocobalamin and a genetic predisposition to the development of SANS [7].
Management
Different approaches are needed to manage SANS. Astronauts undergo rigid testing to check for enzymatic pathway deficiencies described above and efforts made to replace vitamin deficiencies such as folate. In some cases, acetazolamide is utilised to lower the intracranial pressure [8]. Factors such as nutrition, exercise regimes to mimic terrestrial gravitational environments during spaceflight, and special glasses called ‘space anticipation glasses’ are offered to astronauts onboard the ISS in attempts to mitigate hyperopic shifts during LDSF [4].
Acetazolamide has been shown to provide some benefit in lowering CSF pressures in patients with evidence of raised intracranial pressures. This report suggested a regimen of 500mg and 250mg for six and two weeks with repeat LP pressures decreasing from 28 to 19 post LDSF [8]. Widespread consensus on its clinical use during spaceflight remains debatable [8].
Astronauts should be followed up with visual field testing within 72 hours of returning from spaceflight to assess the role of optic disc oedema if present in the formation of blind spots. Also, assessments of OCT retinal nerve fibre layer (RNFL) thickness at regular intervals after spaceflight may shed light on the progression and impact of chronic optic disc oedema.
It is important to note that even after return to earth gravity, clinical signs of SANS may still be visible in the long term, such as elevated CSF opening pressures, optic disc oedema and choroidal folds [9].
Complications
The evidence shows a lack in consistency pertaining to time taken with return of normal visual function following SANS. However, there have been no reported cases of permanent visual loss in ISS crew members following the development of this condition [4].
TAKE HOME MESSAGE
-
SANS is a unique space travel neuro-ocular disease that has no known terrestrial analogue. SANS may pose a potential obstacle to future space, lunar, or Martian missions.
-
Characteristic features of SANS include hyperopic displacement, optic nerve oedema, choroidal folds, flattening of the globe and retinal nerve fibre layer infarcts.
-
The aetiology underlying SANS is not fully understood and remains the subject of much debate. There are several potential mechanisms including cephalic fluid shift, vascular and lymphatic congestion, inflammation, ischaemia, and environmental factors.
-
Acetazolamide may be utilised in the medical management of SANS however there is no guideline or widespread consensus to its use.
-
Long-term permanent visual loss has not been demonstrated in patients who developed SANS.
References
1. Wostyn P, De Deyn PP. Optic nerve sheath distention as a protective mechanism against the visual impairment and intracranial pressure syndrome in astronauts. Invest Ophthalmol Vis Sci 2017;58(11):4601-2.
2. Paez YM, Mudie LI, Subramanian PS. Spaceflight associated neuro-ocular syndrome (SANS): a systematic review and future directions. Eye Brain 2020;12:105-17.
3. Yang JW, Song QY, Zhang MX, et al. Spaceflight-associated neuro-ocular syndrome: a review of potential pathogenesis and intervention. Int J Ophthalmol 2022;15(2):336.
4. Mader TH, Gibson CR, Pass AF, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology 2011;118(10):2058-69.
5. Kramer LA, Sargsyan AE, Hasan KM, et al. Orbital and intracranial effects of microgravity: findings at 3-T MR imaging. Radiology 2012;263(3):819-27.
6. Lee AG, Mader TH, Gibson CR, et al. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: a review and an update. npj Microgravity 2020;6(1):1-10.
7. Smith S, Zwart S, Heer M. Human Adaptation to Spaceflight: The Role of Nutrition. Washington, D.C, USA; United States Government Publishing Office; 2014.
8. Stenger MB, Tarver WJ. Risk of spaceflight associated neuro-ocular syndrome (SANS). Available from:
https://humanresearchroadmap.
nasa.gov/evidence/reports/SANS.pdf?
rnd=0.434276635495143
Last accessed April 2022.
9. Mader TH, Gibson CR, Pass AF, et al. Optic disc edema in an astronaut after repeat long-duration space flight. J Neuroophthalmol 2013;33(3):249-55.
Declaration of competing interests: None declared.
Click the box below for our interview with Captain Tyson J Brunstetter,
Aerospace/Research Optometrist at the NASA Johnson Space Center, on SANS.
COMMENTS ARE WELCOME











