Never ignore the small things’…someone once said. There is no doubt blepharitis is one of the most common eye conditions encountered daily, but with the typical pressures of a busy outpatient department, the management of more obvious, sight-threatening conditions necessarily demands the majority attention from professionals. However, the prevalence and impact of this common condition is clearer than ever before, giving rise to some interesting observations particularly regarding the pathophysiology of evaporative dry eye.
How common is blepharitis?
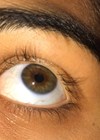
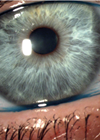
Blepharitis is the broad term to describe any inflammation of the eyelids and their margins, but when talking to patients most eye care professionals tend to use this term when describing simple anterior blepharitis – the greasy or dry crusts gathering around the base of the lashes that require long-term regimes of lid hygiene. The most common cause of posterior blepharitis, meibomian gland dysfunction (MGD) is often discussed separately, when in reality the two conditions go hand-in-hand.

Figure 1: Signs of MGD are easily overlooked.
This leads to some conflicting results in the literature when looking at prevalence figures. Blepharitis apparently accounts for one in 20 eye problems reported to GPs [1], but perhaps we can get a better idea of prevalence for the UK amongst apparently normal patients attending optometric practice for routine eye examinations [2,3] where some degree of blepharitis is reported in around 40% in middle-age, rising to 66% amongst the over 60s. Population based studies show variations between 3-70%, with the higher prevalence observed in Asian populations [4]. But this is where it gets interesting, when you examine office populations complaining of symptoms when working with computers – abnormal meibum secretions are observed in at least 74% [5] of this relatively younger group, with significant correlations between symptoms and signs of MGD [6]. It is interesting because this is a group of patients who are very likely to have dry eye but don’t naturally get anterior blepharitis – more about this later.
There is a clear link which is now established between MGD and dry eye: it is the most common cause of dry eye [7], with typically over 80% of dry eye clinic patients demonstrating signs of MGD [8]. This has direct implications for the modern management of dry eye, where traditionally ocular lubricants are first line therapy, in contrast to the research, which suggests that eight out of 10 dry eye sufferers need to manage eyelid inflammation as their first priority.
How does someone develop blepharitis in the first place?
Anterior blepharitis can result from simple poor hygiene resulting in a bacterial (usually staphylococcal) disease process, or it can be a seborrheic disease process, like dandruff of the scalp. The prevalence of blepharitis amongst people with ocular rosacea, psoriasis and androgen deficiency is significantly higher: for example, MGD affects more than 80% of rosacea patients [9]. The meibomian glands, as modified sebaceous glands, are mainly under hormonal (androgen) control, so conditions such as Parkinson’s and medications for benign prostrate hyperplasia are strongly associated with MGD.
In the absence of systemic risk factors, the classical pathophysiology of MGD relates directly to the degradation of meibum secretions (via saponification) by the lipases produced by Staphylococcal bacteria on the lid margin. The end result is local inflammation in and around the gland ducts, ultimately leading to MGD. Interestingly, saponification is the very process that will occur when diluted baby shampoo is applied to the lid margins. Whilst this might be the traditional regimen indicated by current UK clinical guidelines, its benefits are questionable; indeed, there are no published studies that support its use over other methods of lid hygiene.
But what about people with MGD and clean eyelid margins? For example, how does MGD develop in a young office worker with average bacterial load on the lid margins? And in Sjogren’s syndrome, there appears to be a higher incidence of MG dropout [10]. The explanation is more recent and stems from an eloquent theory put forward by Professor Bron and colleagues: they proposed that where poor tear secretion, excessive evaporation, or infrequent blinking leads to diminished tear volume, there will be a steep solute gradient in the meniscus at the lid margins.
The hyperosmolarity will be greatest at the leading edge of the meniscus, in close proximity to the meibomian gland orifices, and they hypothesised that this could play a role in the initiation of MGD by delivering hyperosmolar and inflammatory stresses to the terminal ducts and orifices of the glands [11]. A recent mouse model would seem to support this by demonstrating that meibomian gland function is impaired in a desiccating environment [12]. This understanding might contribute to the higher prevalence of MGD amongst our digitally-native patients.
There has been a resurgence of interest, particularly amongst optometrists, in the relevance of the common mites, Demodex folliculorum and Demodex brevis, as a cause of chronic blepharitis. Their prevalence in humans is endemic and increases with age; thought to be 100% after 70 years of age, but there are statistical associations reported between mite density and rosacea, facial itching and blepharitis. What remains unclear is pathogenic mechanism – they could be coincidental or causative, and it could be that there exists certain, more virulent forms with symbiotic bacteria flora that interact in a specific way in some patients [13,14]. There are very few practical options for completely eradicating these mites, so regular lid hygiene remains the mainstay of management in all but severe, recalcitrant cases.
Advances in assessing the patient with MGD
Recent advances in infra-red (IR) imaging equipment has seen several devices come to market for imaging the meibomian glands, which are only visible using IR or trans-illumination. However, the clinical norms across age groups are still being investigated, and available grading scales remain unvalidated. Of importance is the observed natural age-related deterioration in the glands [15], which may in part explain the increased prevalence of dry eye in older people.
“Where poor tear secretion, excessive evaporation, or infrequent blinking leads to diminished tear volume, there will be a steep solute gradient in the meniscus at the lid margins.”
Diagnostic and therapeutic gland expression is favoured by many clinicians, with an increasing range of surgical instruments adapted for this purpose, but the effect of any local trauma to the tissue is actually unknown.
Modern physical management of anterior blepharitis and MGD
Keeping eyelids clean is the number one priority, to minimise the bacterial load, reducing risk of infection and avoiding long-term complications. The detrimental effects of using baby shampoo for lid hygiene regimes are two-fold: the immediate destabilisation of the tear film and the degradation of the meibum lipids themselves, leading to MG obstruction / inflammation over time. There is a plethora of alternative products available to buy over the counter for gentle eyelid cleansing, although many still contain soap. For patients, gentle lid massage to encourage the meibum to flow should follow or accompany cleansing – an excellent review article by Benitez-Del-Castillo (2012) discusses the importance of demonstrating this action [16]. The recommended cleansing regime is twice in a day (b.i.d) for 21 days followed by maintenance of once a day (o.i.d) [17].
Warming the meibomian glands through closed eyelids using warm compresses has been the mainstay of management for decades, but is laborious for this chronic condition and too imprecise in temperature to be effective. Dysfunction in the gland fundamentally alters the lipid composition and ultimately raises the melting point by around 3°C [18] to around 35°C [19] – so effective warming, sufficient to melt meibum, requires around 39-40°C for at least six minutes: this is impossible to achieve with the average face cloth. Published randomised controlled trials support the clinical effectiveness of two alternative products patients can be directed towards [20,21].
Aside from the obvious mechanical benefits of clearing obstructions in the gland due to thickened secretions, more recent research shows that warming changes the lipids fundamentally [22], and there is evidence to suggest that long-term warming therapy actually curtails excess phospholipase activity in MGD [23]. There might also be an emerging role for heat therapy in the management of neuropathic ocular pain in dry eye [24].
Novel ‘mechanical’ approaches include lid margin debridement, intense pulsed light therapy (IPL), and thermal pulsation, all of which have published studies showing degrees of clinical improvement in MGD. Where there is keratinisation of the muco-cutaneous junction lid margin debridement appears to improve symptoms and MG function [25,26].
How much impact does blepharitis have?
Whilst blepharitis might seem a ‘small thing’ in ophthalmology, its impact is far-reaching. Clinically, if left untreated the local effects of blepharitis can lead to conjunctivitis, lid scarring, trichiasis, madarosis, and ultimately, corneal involvement leading to loss of vision (blepharokeratoconjunctivitis). Whilst not indicating cause, the condition is also associated with a significantly higher incidence of anxiety and depression [27], ulcerative colitis and irritable bowel syndrome (IBS) [28]. Some have suggested that a chronic pro-inflammatory state may be common amongst these conditions. There is also an economic impact – blepharitis can be a risk factor for endophthalmitis after cataract surgery [29]; implementing regimes for lid hygiene prior to surgery appears to bring considerable cost savings for the NHS by avoiding cancellations [30].
Summary
Blepharitis, both anterior and posterior is very common but probably under-diagnosed and under-managed in the UK. Current clinical guidelines for management would benefit from updating in the light of more recent understanding.
References
1. Blepharitis. NHS Choices.
http://www.nhs.uk/conditions/blepharitis/
Pages/Introduction.aspx
Last accessed August 2015.
2. Hom MM, Martinson JR, Knapp LL, Paugh JR. Prevalence of Meibomian gland dysfunction. Optom Vis Sci 1990;67(9):710-2.
3. Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf 2009;7(2 Suppl):S1-14.
4. Schaumberg DA, Nichols JJ, Papas EB, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci 2011;52(4):1994-2005.
5. Fenga C, Aragona P, Cacciola A, et al. Meibomian gland dysfunction and ocular discomfort in video display terminal workers. Eye (Lond) 2008;22(1):91-5.
6. Wu H, Wang Y, Dong N, et al. Meibomian gland dysfunction determines the severity of the dry eye conditions in visual display terminal workers. PLoS One 2014;9(8): e105575.
7. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci 2011;52(4):1922-9.
8. Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea 2012;31(5):472-8.
9. Patiño-Rodríguez BE, Rodríguez-García A, Díaz JC, et al., External ocular surface changes in ocular rosacea patients. Rev Mex oftalmol 2012;86:86-96.
10. Menzies KL, Srinivasan S, Prokopich CL, Jones L. Infrared imaging of meibomian glands and evaluation of the lipid layer in Sjögren’s syndrome patients and non dry eye controls. Invest Ophthalmol Vis Sci 2015;56(2):836-41.
11. Bron AJ1, Yokoi N, Gaffney EA, Tiffany JM. A solute gradient in the tear meniscus. II. Implications for lid margin disease, including meibomian gland dysfunction. Ocul Surf 2011;9(2):92-7.
12. Suhalim JL, Parfitt GJ, Xie Y, et al. Effect of desiccating stress on mouse meibomian gland function. Ocul Surf 2014;12(1):59-68.
13. Elston DM. Demodex mites: facts and controversies. Clin Dermatol 2010;28(5):502-4.
14. Chen W, Plewig G. Are Demodex mites principal, conspirator, accomplice, witness or bystander in the cause of rosacea? Am J Clin Dermatol 2015;16(2):67-72.
15. Nien CJ, Massei S, Lin G, et al. Effects of age and dysfunction on human meibomian glands. Arch Ophthalmol 2011;129(4):462-9.
16. Benitez-Del-Castillo JM. How to promote and preserve eyelid health. Clin Ophthalmol 2012;6:
1689-98.
17. Guillon M, Maissa C, Wong S. Symptomatic relief associated with eyelid hygiene in anterior blepharitis and MGD. Eye Cont Lens 2012;38(5):306-12.
18. Ong BL, Larke JR. Meibomian gland dysfunction: some clinical, biochemical and physical observations. Ophthalmic Physiol Opt 1990;10(2):144-8.
19. Terada O, Chiba K, Senoo T, Obara Y. Ocular surface temperature of meibomia gland dysfunction patients and the melting point of meibomian gland secretions. Nihon Ganka Gakkai Zasshi 2004;108(11):690-3.
20. Bilkhu PS, Naroo SA, Wolffsohn JS. Randomised masked clinical trial of the MGDRx eyebag for the treatment of meibomian gland dysfunction-related evaporative dry eye. Br J Ophthalmol 2014;98(12):1707-11.
21. Sim HS, Petznick A, Barbier S, et al. A randomized, controlled treatment trial of eyelid-warming therapies in meibomian gland dysfunction. Ophthalmol Ther 2014;3(1-2):37-48.
22. Leiske DL, Leiske CI, Leiske DR, et al. Temperature-induced transitions in the structure and interfacial rheology of human meibum. Biophys J 2012;102(2): 369-76.
23. Longitudinal changes in tear fluid lipidome brought about by eyelid-warming treatment in a cohort of meibomian gland dysfunction. J Lipid Res 2014;55(9):1959-69.
24. Galor A, Levitt RC, Felix ER, et al. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond) 2015;29(3):301-12.
25. Korb DR, Blackie CA. Debridement-scaling: a new procedure that increases Meibomian gland function and reduces dry eye symptoms. Cornea 2013;32(12):1554-7.
26. Ngo W, Caffery B, Srinivasan S, Jones LW. Effect of lid debridement-scaling in Sjögren syndrome dry eye. Optom Vis Sci 2015;92(9):316-20.
27. Chiang CC, Lin CL, Tsai YY, et al. Patients with blepharitis are at elevated risk of anxiety and depression. PLoS One 2013;8(12):e83335.
28. Nemet AY, Vinker S, Kaiserman I. Associated morbidity of blepharitis. Ophthalmology 2011;118(6):1062-8.
29. Speaker MG, Milch FA, Shah MK, et al. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology 1991;98(5):639-49.
30. Stead RE, Sturat A, Keller J, Subramaniam S. Reducing the rate of cataract surgery cancellation dues to blepharitis. Eye (Lond) 2010;24:742.
Declaration of Competing Interests: Thea Pharmaceuticals supply a range of eye care medicines, medical devices and lid hygiene products, including those for dry eye and blepharitis.
COMMENTS ARE WELCOME