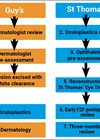
Novel anti-cancer therapies have led to significant advancement in cancer treatment, however, they can be associated with external eye complications. It is important to be mindful of such adverse effects during assessment of patients enrolled in clinical trials.
Annually, approximately 330,000 individuals are diagnosed with cancer in the UK, with this number predicted to exceed 400,000 by 2030 due to an ageing and expanding population [1]. Despite an increasing burden of cancer diagnoses, mortality from cancer is, conversely, falling [1]. This is thanks in part to an emphasis on early diagnosis and early specialist input, but also due to revolutionary new treatment that has emerged over the last two decades [1,2].
Targeted anti-cancer treatments, which inhibit the growth, progression and spread of cancer through their action on specific molecular targets have contributed to huge leaps in survival rates [3]. These novel agents represent an important area of anti-cancer research [3]. Unfortunately, accompanying these significant gains is a plethora of ocular adverse effects, therefore, it is crucial that all eye specialists are equipped with the skills to identify and treat these complications.
What are targeted anti-cancer treatments?
Targeted anti-cancer treatments differ from conventional chemotherapy through their ability to act on specific targets associated with a cancer, rather than all rapidly dividing cells [3]. The National Cancer Institute broadly classifies these as [3]:
Hormone therapies
Breast, ovarian, endometrial and prostate cancer, amongst others, are dependent on hormones such as oestrogen and testosterone for proliferation and spread, with over 75% of breast cancers oestrogen-receptor positive [3]. Therapies that directly or indirectly target these include aromatase inhibitors, such as letrozole and selective oestrogen receptor modulators (SERMs), such as tamoxifen [2,3].
Monoclonal antibodies
Immune checkpoint inhibitors (ICIs), such as pembrolizumab and ipilimumab, are monoclonal antibodies whose action leads to an upregulated immune response against tumours [2]. Despite the eye’s relative immune privilege, immune-related adverse events (irAEs) may occur in any ocular structure, affecting 1% of patients on ICIs [4]. Antibody-drug conjugates (ADCs) target specific antigens on a malignant cell, aiming to spare healthy cells from apoptosis [2].
Cancer growth inhibitors
Cellular growth and division are dependent on the action of growth factors and chemical messengers, such as tyrosine kinases [3,5]. Inhibition of these targets, as well as mTOR, BRAF and MEK proteins, has proven effective in treatment of a variety of cancers [5]. Dysregulated angiogenesis is a cornerstone of malignancy. Medications such as pazopanib and azitinib target vascular endothelial growth factor receptors (VEGFR), a key stimulant for new vessel growth [2].
PARP inhibitors
Inhibition of poly ADP-ribose polymerases (PARPs), which are responsible for repairing damaged DNA and thus evasion of apoptosis, is an important treatment for breast and ovarian cancer [2].
Apoptosis-inducers
Therapeutic agents such as everolimus and boretezomib target key points in the signalling pathway to induce apoptosis in a malignant cell [2].
What is the role of the ophthalmologist?
If ocular adverse events are not recognised and treated promptly, they can have a significant impact on quality of life and may contribute to patients discontinuing potentially lifesaving treatment [6].
“Patients with baseline pathology or history of ocular symptoms are at an increased risk of developing ocular complications”
Patients with baseline pathology or history of ocular symptoms, such as dry eyes, are at an increased risk of developing ocular complications over the course of treatment [6]. Incorporating a structured approach to assessment is crucial, as patients are significantly more likely to report symptoms with focused questioning [7]. Slit-lamp examination is important to identify severe pathology which may be asymptomatic or present non-specifically [2,6]. For example, during the DREAMM-2 trial, some patients with severe keratopathy or corneal ulcers were only detected on routine ocular assessment [7].
Structured slit-lamp examination
Adopting a systematic approach to examination is key to identifying common anti-cancer therapy-related adverse effects. Whilst this list is not exhaustive, it serves as a basis for eye specialists examining the external eye on novel therapeutic agents.
Conclusion
Targeted anti-cancer therapies have led to significant improvements in cancer-related mortality since their introduction. Many of these agents suppress cellular growth and stimulate apoptosis by targeting cell-signalling pathways or promoting an upregulated immune response. This can result in off-target adverse effects in all body systems, importantly including the eye. It is crucial that these patients are monitored regularly with a systematic structured ocular examination, and that eye specialists are equipped with the knowledge and skills to identify common treatment-related complications.
TAKE HOME MESSAGE
-
If in doubt about the aetiology of external eye complications – consider current or previous anti-cancer therapy.
-
Adopt a systematic approach to examination as some pathology may be asymptomatic.
-
Most complications from targeted anti-cancer therapy can be managed without treatment cessation, however, prompt recognition and treatment are essential.
References
1. Creighton H, Beach B, Bamford SM. Rethinking cancer: The BigC: Quantifying the social and economic impact. International Longevity Centre. September 2015:
https://ilcuk.org.uk/wp-content/
uploads/2018/10/Rethinking
-Cancer-The-big-C.pdf
2. Fortes BH, Tailor PD, Dalvin LA. Ocular Toxicity of Targeted Anticancer Agents. Drugs 2021;81(7):771-823.
3. Targeted Cancer Therapies. National Cancer Institute. 2021:
https://www.cancer.gov/about-cancer/
treatment/types/targeted-therapies/
targeted-therapies-fact-sheet
4. Park RB, Jain S, Han H, Park J. Ocular surface disease associated with immune checkpoint inhibitor therapy. Ocul Surf 2021;20:115-29.
5. Cancer Growth Blockers. Cancer Research UK. 2021
https://www.cancerresearchuk.org/
about-cancer/cancer-in-general/treatment/
targeted-cancer-drugs/types/
cancer-growth-blockers
6. Wahab A, Rafae A, Mushtaq K, et al. Toxicity of Belantamab Mafodotin, an Oncological Perspective of Management in Relapsed and Refractory Multiple Myeloma. Front Oncol 2021;11:678634.
7. Kruft B, Nelson LA, Stewart JA, Stewart WC. Adverse event reporting. Ophthalmology 2007;114(7):1420.
8. Anforth R, Fernandez-Peñas P, Long GV. Cutaneous toxicities of RAF inhibitors. Lancet Oncology 2013;14(1):e11-e18.
9. Breccia M, Gentilini F, Cannella L, et al. Ocular side effects in chronic myeloid leukemia patients treated with imatinib. Leukemia Research 2008;32(7):1022-5.
10. Heinrich MC, Jones RL, von Mehren M, et al. Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial. Lancet Oncology 2020;21(7):935-46.
11. Pascual J, Marcén R, Ortuño J. Clinical experience with everolimus (Certican): optimizing dose and tolerability. Transplantation 2005;79(9):S80-S84.
12. Schear MJ, Rodgers R. A case of everolimus-induced eyelid edema. Ophthalmic Plast Reconstr Surg 2018;34(1):e21-e22.
13. Narala R, Reddy SA, Mruthyunjaya P. Giant cell arteritis manifesting as retinal arterial occlusion and paracentral acute middle maculopathy in a patient on pembrolizumab for metastatic uveal melanoma. Am J Ophthalmol Case Rep 2020;20:100891.
14. Caberry WY, Yau M, Mezey N, et al. Neuro-Ophthalmic complications of immune checkpoint inhibitors: a systematic review. Eye and Brain 2020;12:139.
15. Winthrop KL, Melmed GY, Vermeire S, et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis 2018;24(10):2258-65.
16. Kobak S. Tofacitinib-induced Ramsay-Hunt syndrome in a patient with rheumatoid arthritis. Curr Drug Safety 2021;16(1):107-9.
17. Chatziralli I, Sergentanis T, Zagouri F, et al. Ocular surface disease in breast cancer patients using aromatase inhibitors. Breast J 2016;22(5):561-3.
18. Zhang G, Basti S, Jampol LM. Acquired trichomegaly and symptomatic external ocular changes in patients receiving epidermal growth factor receptor inhibitors: case reports and a review of literature. Cornea 2007;26(7):858-60.
19. An S, Raju I, Surenkhuu B, et al. Neutrophil extracellular traps (NETs) contribute to pathological changes of ocular graft-vs.-host disease (oGVHD) dry eye: Implications for novel biomarkers and therapeutic strategies. Ocul Surf 2019;17(3):589-614.
20. Méndez-Martínez S, Calvo P, Ruiz-Moreno O, et al. Ocular adverse events associated with MEK inhibitors. Retina 2019;39(8):1435-50.
21. Csaky KG, Dugel PU, Pierce AJ, et al. Clinical evaluation of pazopanib eye drops versus ranibizumab intravitreal injections in subjects with neovascular age-related macular degeneration. Ophthalmology 2015;122(3):579‑88.
22. Dulley P. (1999). Ocular adverse reactions to tamoxifen – a review. Ophthalmic Physiol Opt 1999;19(Suppl1):S2-9.
23. Zhou Z, Sambhav K, Chalam KV. Erlotinib-associated severe bilateral recalcitrant keratouveitis after corneal EDTA chelation. Am J Ophthalmol Case Rep 2016;4:1-3.
24. Farooq AV, Degli Esposti, S, Popat R, et al. Corneal epithelial findings in patients with multiple myeloma treated with antibody–drug conjugate belantamab mafodotin in the pivotal, randomized, DREAMM-2 study. Ophthalmol Ther 2020;9(4):889-911.
(All links accessed December 2021).
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME