Case presentation
We present the case and discussion of a 46-year-old Caucasian male who complained of immediate reduced vision following complicated neck dissection including a total laryngopharyngectomy, free flap reconstruction for a T4N3 squamous cell carcinoma (SCC) of the left hypopharynx and supraglottis. Postoperative day one examination findings were as shown in Table 1.
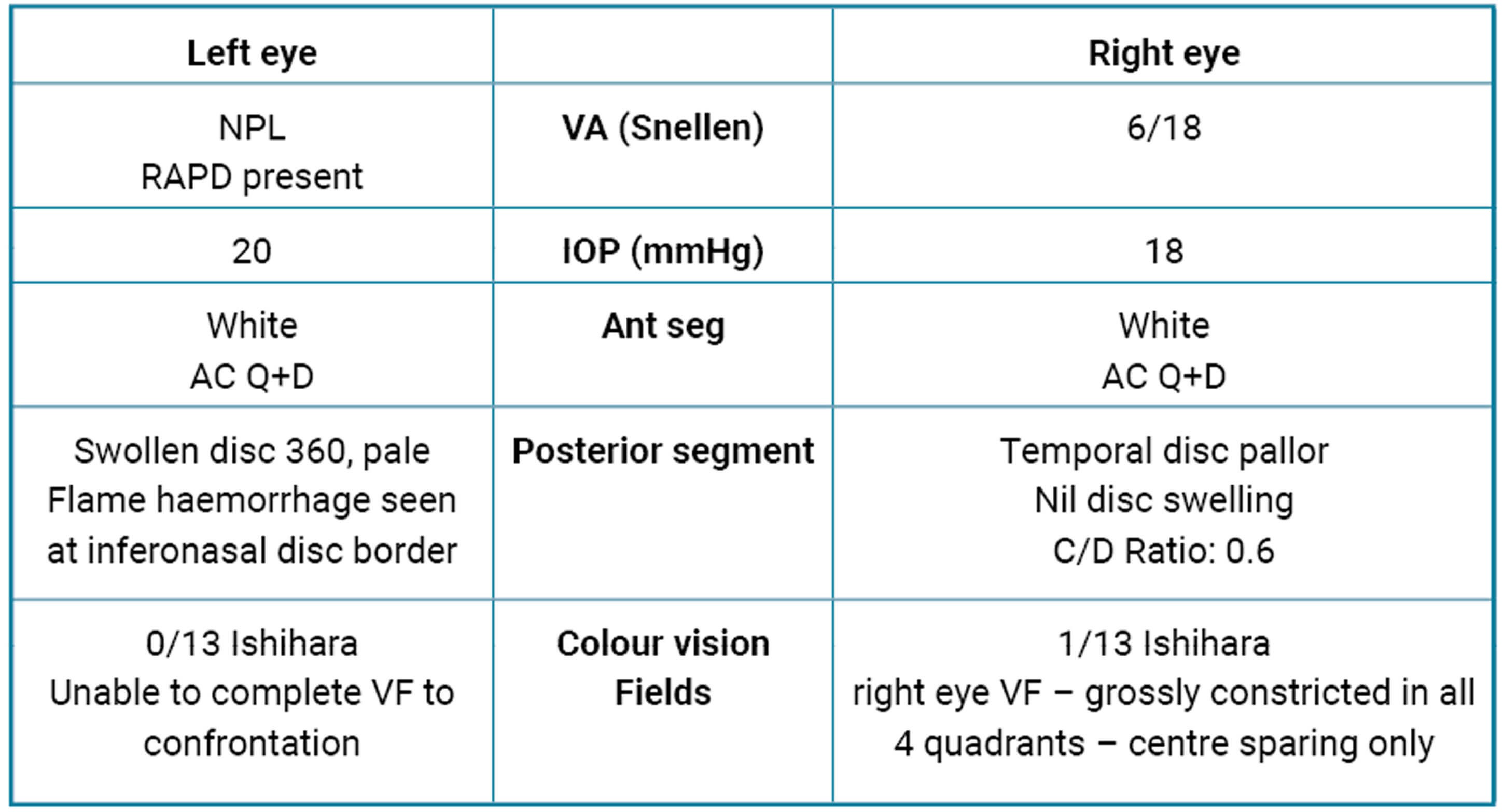
Table 1: Examination findings on day one.
Further history excluded any significant past medical history, except for previous illicit drug use prior to incarceration two years ago. No known drug allergies were reported and there was no significant family history. Total surgical time documented exceeding 10 hours with intraoperative hypotensive episodes and catecholamine requirements. Investigations including baseline and postoperative bloods are shown below. Given this presentation, neuroimaging of the head and neck was completed.
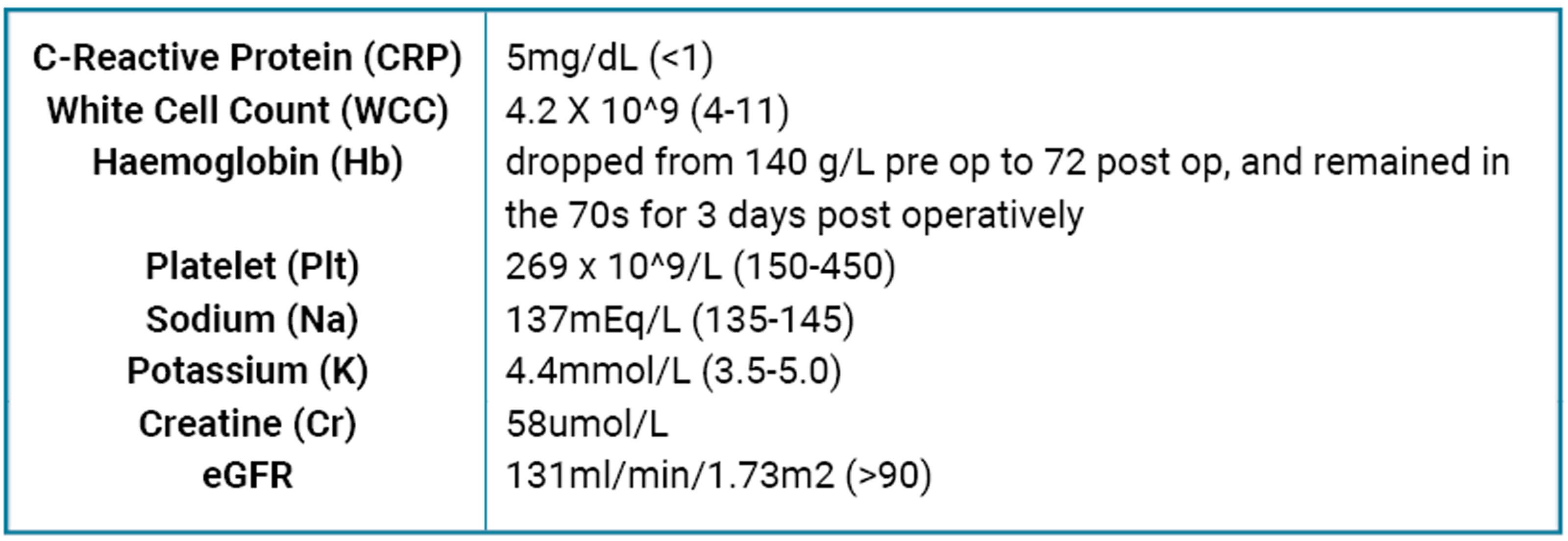
Table 2: Blood results during inpatient stay perioperatively.
The final MRI Report – Head and Neck
The final MRI Report – Head and NeckHistory of previous laryngopharngngectomy and free flap reconstruction for SCC noted. Some of the sequences are markedly movement degraded, reducing the sensitivity of the study. There is no mass or focus of pathological enhancement. No definite signal abnormality is seen in relation to the optic nerves and chiasm, however these appear slender. An equivocal focus of enhancement in relation to the left pre-chiasmatic optic nerve could be vascular. No compressive lesion is seen in relation to the anterior visual pathways.
Incidental note of bilateral mastoid effusions. Post-treatment changes are seen within the neck bilaterally.
Opinion
-
No evidence of intracranial metastases or demyelination.
-
The optic nerves and chiasm appear slender. No signal abnormality or compressive lesion is seen in relation to the optic nerves or chiasm.
Figure 1 highlights differentials of a working diagnosis of the optic neuropathy with the likelihood of giant cell arteritis in this case considered low given the patient demographics, and a lack of clinical symptoms reported by the patient in addition to normal inflammatory markers. In this case, given the surgical history and postoperative prolonged anaemia with otherwise normal blood results and normal MRI imaging, a diagnosis of non-arteritic ischemic optic neuropathy (NAION) was made.
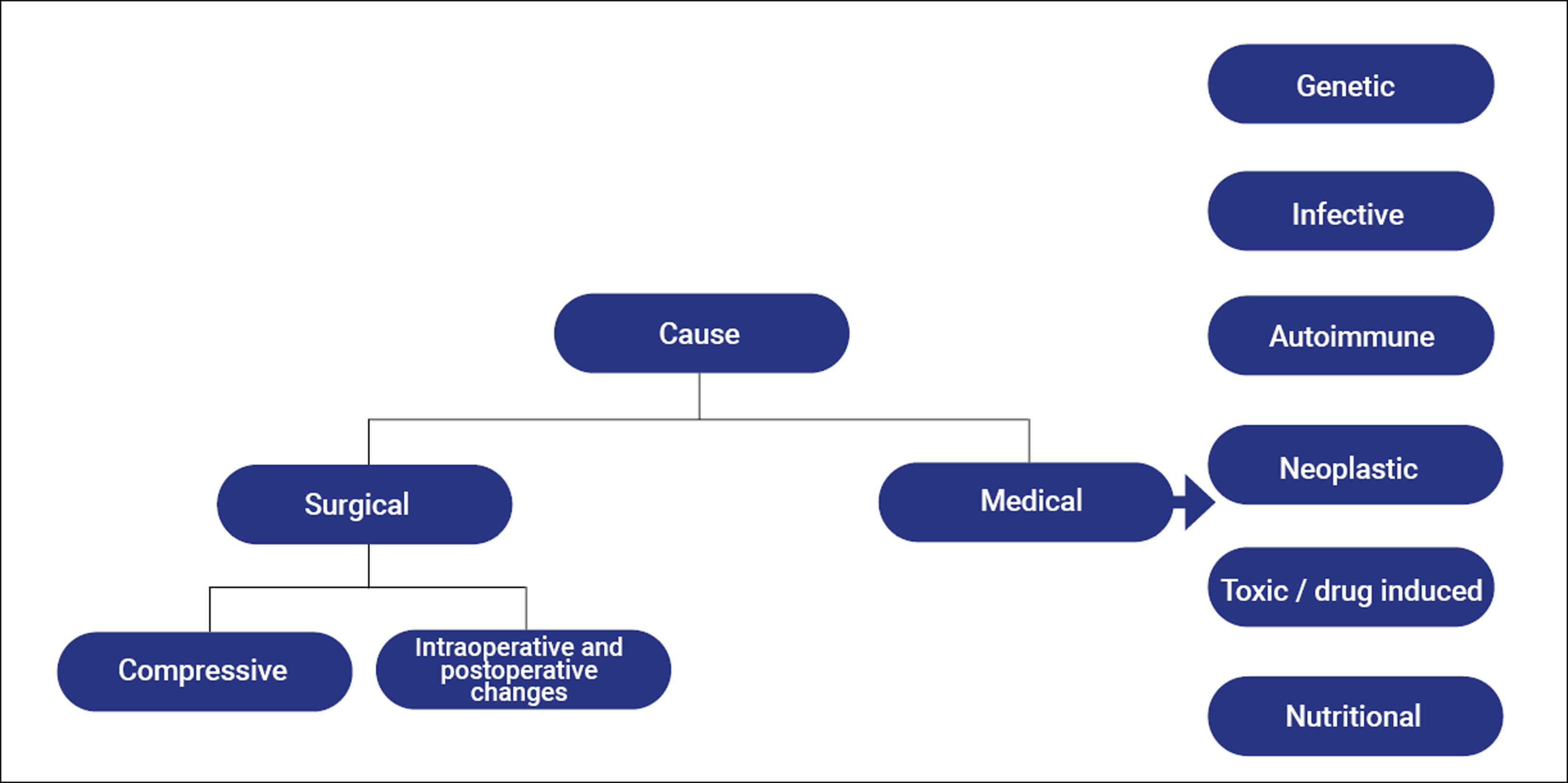
Figure 1: Differentials of a working diagnosis of optic neuropathy.
Non-arteritic ischemic optic neuropathy
NAION is one of the commonest causes of optic neuropathy including glaucoma and arteritic optic neuropathy [1]. Ischaemic optic neuropathy (ION) can be split into anterior (AION) and posterior ischaemic optic neuropathy (PION). By definition, AION involves the anterior 1mm segment of the optic nerve head (the optic disc) and therefore will result in visible disc swelling on examination. PION occurs due to ischaemia to any part of the optic nerve posterior to the optic disc. Thus on examination in PION, there will be no visible disc oedema. The diagnosis of NAION is a clinical one which does not always require further testing in those who present with classical history and findings.
Clinical presentation
NAION occurs more commonly in the Caucasian population and there is no documented gender predilection. The mean onset is between 57–60 years of age [2]. Visual acuity (VA) can vary. Patients tend to describe sudden painless monocular loss of VA [3], however in the case of acuities of no perception of light (NPL) it is important to suspect and rule out arteritic AION. Visual field defects are usually inferior altitudinal but can also be central, para-central or arcuate [4]. Dyschromatopsia is a sensitive sign of optic nerve dysfunction which can be proportional to the NAION. Of note, colour vision can be intact if the central fibres are spared [5]. Diffuse or more commonly segmental disc swelling tends to be hyperaemic and rarely pallid. The size of the optic disc of the other eye should be documented as a small or absent cup is a risk for NAION. Table 3 outlines typical characteristic features in arteritic vs. non-arteritic AION.
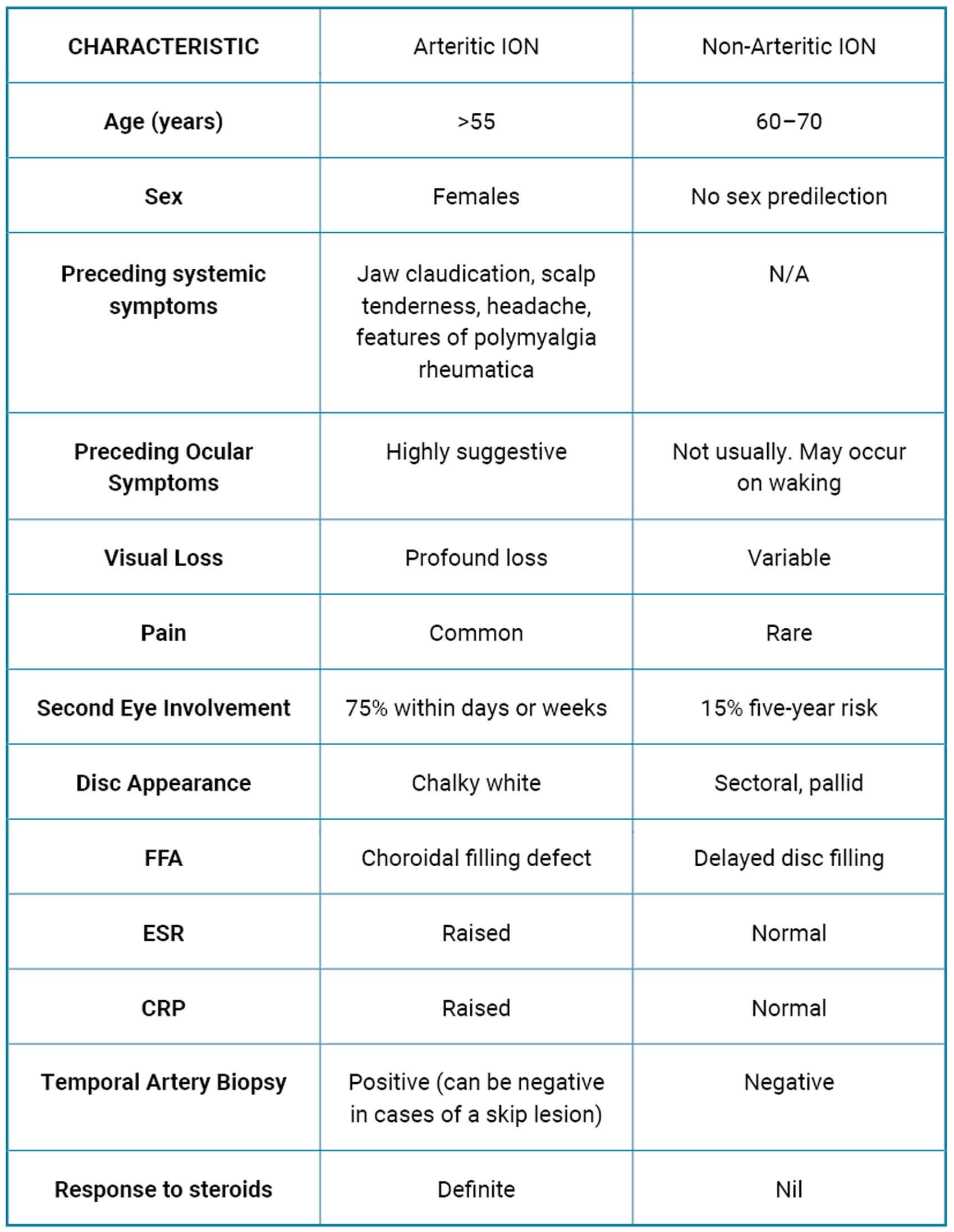
Table 3: Characteristic features of arteritic vs. non-arteritic ION.
Pathophysiology
In the case of post-surgical visual loss in NAION, a multifactorial cause has been hypothesised. Hypotension and anaemia from intraoperative blood loss and anesthesia combined with nerve oedema from venous related congestion following surgically induced loss of the internal jugular system results in reduced circulation to the posterior optic nerve [6,7]. Additional suggested hypotheses in NAION include hypoperfusion, autoregulatory failure, nocturnal hypotension and vascular changes including spastic and thromboembolic changes [1,4].
Investigations
Importantly, in all patients 50 years and older it remains critical to rule out giant cell arteritis (GCA), which if missed can lead to catastrophic irreversible visual loss, life-threatening complications, and medico-legal issues for the clinician if missed. Investigations involve blood inflammatory markers including a C-reactive protein and erythrocyte sedimentation rate. Additional investigations depending on the likelihood of GCA include ultrasound or temporal artery biopsy. The British Society of Rheumatology have updated their guidelines on the diagnosis and treatment of GCA [8]. Following this, other specific aetiologies which should be ruled out are seen in Figure 1. Alternative causes should be considered in the presence of any atypical features including:
- Age <40y
- Painful loss of vision
- Bilateral simultaneous onset or rapid sequential loss
- Lack of optic disc oedema
- Optic disc oedema lasting >4 weeks
- Atypical visual field defects
- Absence of an relative afferent pupillary defect (RAPD)
- Lack of vasculopathic risk factors
- Large cup:disc ratio
- Macular star.
Thorough history taking should include a discussion of risk factors (hypertension, hypercholesterolaemia, diabetes, sleep apnoea) and medication history (including use of medications including phosphodiesterase type 5 inhibitors, inferons, illicit drug use, smoking). NAION remains a clinical diagnosis and generally further investigations are not always indicated. Features of obstructive sleep apnoea that should be questioned include day-time somnolence, choking and snoring at ny7ytight. Patients should be referred for overnight sleep studies for further investigation. Neuroimaging (MRI brain and orbits) is generally not necessary in typical cases but should be obtained to exclude an inflammatory or compressive cause, particularly in those with any atypical features.
Management and prognosis
Currently, no effective treatment for NAION exists. Visual loss has been previously documented as a rare complication of head and neck mass surgery. There are currently 15 documented clinical cases of visual loss following head and neck surgery in which the key aetiologies included intraoperative hypotension, blood loss and venous congestion [4]. Within the case series report, visual loss manifested immediately postoperatively in all cases except for one case following the fourth day of surgery. Suggested techniques to reduce the risk of this include cautious vasopressor use [9], perioperative blood transfusion [10], and careful intraoperative patient positioning of the head at heart level in a forward position [11]. Following from the case presentation above, both pre-, peri- and postoperative management and close monitoring are considered important for the prevention and monitoring of patients at risk of visual loss. An argument exists to consider notifying patients of the risk of operative related visual loss as part of the standard consenting procedure.
Progressive forms of NAION have also previously been reported [1]. The typical course of NAION stabilises after a few months. Visual field defects are less likely to improve compared to visual acuity. There is a 15% five-year chance of fellow eye involvement. The highest-level evidence that currently exists for the treatment of NAION includes the Ischaemic Optic Neuropathy Decompression Trial [3] – a prospective randomised trial which did not find optic nerve fenestration effective for NAION. Other studies have involved medical intervention including the use of steroids (systemic, periocular and intraocular), oral levodopa, aspirin, topical brimonidine as well as other surgical interventions including radial optic neurotomy.
Many of the studies are limited by the small sample sizes, lack of prospective design or lack of blinding. Subtle structural improvements and faster resolution of optic disc oedema has been noted following a double blind randomised control trial (RCT) involving oral corticosteroids in the treatment of NAION. There was a significant improvement in best corrected VA (BCVA) at six months however no significant difference remained in final BCVA [12]. No current clinical guidelines exist for the treatment of NAION and the use of corticosteroids remains controversial.
TAKE HOME MESSAGES
-
First and foremost exclude arteritic AION in patients presenting with painless loss of vision and swollen optic disc.
-
Exclude any atypical features from history and examination.
-
Consider detailed history including excluding features obstructive sleep apnoea.
-
Consider pre / intra and postoperative optimisation in patients at risk of visual loss from complex head and neck surgery.
References
1. Berry S, Lin W, Sadaka A, Lee A. Nonarteritic anterior ischemic optic neuropathy: Cause, effect, and management. Eye Brain 2017;9:23–8.
2. Lee AG, Brazis PW. Clinical Pathways in Neuro-Ophthalmology: An Evidence-Based Approach. Stuttgart, Germany; Thieme; 2003.
3. Characteristics of patients with nonarteritic anterior ischemic optic neuropathy eligible for the ischemic optic neuropathy decompression trial. Arch Ophthalmol 1996;114(11):1366.
4. Miller NR, Arnold AC. Current concepts in the diagnosis, pathogenesis and management of nonarteritic anterior ischaemic optic neuropathy. Eye (Lond) 2015;29(1):65–79.
5. Kerr NM, Chew SSSL, Danesh-Meyer HV. Non-arteritic anterior ischaemic optic neuropathy: A review and Update. J Clin Neurosci 2009;16(8):994–1000.
6. Krasnikova A, Kreutzer K, Angermair S, et al. Blindness following bilateral neck dissection. A case report and review of the literature. Int J Surg Case Rep 2020;77:201–5.
7. Nazari H, Berry S, Sadaka A, Lee AG. Managing NAION. Specialist 2017;15:201715.
8. Mackie S, Dejaco C, Camellino D, et al. British society for rheumatology guideline on diagnosis and treatment of giant cell arteritis: executive summary. Rheumatology 2020;59(3):487–94.
9. Lee LA. Blindness in the intensive care unit: possible role for vasopressors? Anesth Analg 2005;100(1):192–5.
10. Pazos GA. Blindness after bilateral neck dissection: case report and review. Am J Otolaryngol 1999;20(5):340–5.
11. Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol 2008;145(4):604–10.
12. Hayreh SS. Re: Saxena et al.: Steroids versus no steroids in nonarteritic anterior ischemic optic neuropathy: A randomized controlled trial (Ophthalmology 2018;125:1623–7). Ophthalmology 2019;126(12):e93–4.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME