Part 2: Clinical presentation and treatment (see part 1 here)
Introduction
IgG4-related disease (IgG4-RD) is understood to have a vast clinicopathological spectrum; nearly every organ has had reported involvement. Similarly, IgG4-related ophthalmic disease (IgG4-ROD) is known to affect nearly every part of the eye and adnexae [1].
Clinical Presentation of IgG4-ROD
The lacrimal gland is by far the most commonly reported structure to be affected by pathologically diagnosed IgG4, followed by the trigeminal nerve, extraocular muscles (EOM) and orbital soft tissue [2]. Most other ocular structures have been affected to a much lesser extent [3-10]. While IgG4-ROD may primarily present as an ocular problem, multidisciplinary input is essential, as this is a disease with systemic implications. Extra-ophthalmic involvement is found in up to 75-100% of cases [11,12].
Several studies suggest between 62% (13/21) to 98.4% (221/225) of IgG4-ROD involved the lacrimal gland and up to 69% (49/71) solely involve the lacrimal gland [2,8,13,14]. This correlates to presenting symptoms usually being painless eyelid swelling, xeropthalmia and proptosis [15]. Bilateral involvement of IgG4-ROD is common though not necessarily symmetrical and has been found in up to 93% of patients [5-8,11,16-18]. The natural history of lacrimal gland IgG4 disease has been observed to be similar to other organs with a slow evolvement of lymphoid hyperplasia to significant atrophic fibrosis, though cases of very rapid fibrosis have been reported [10,15]. Bilateral orbital involvement with IgG4 is more highly associated with IgG4-RD elsewhere, compared to unilateral disease; a study demonstrating extra-ophthalmic involvement was present in (69%) 79/114 bilateral cases compared to 29% (20/68) unilateral cases [19]. Extra-ophthalmic organs are commonly local lymph nodes and salivary glands [11,12].
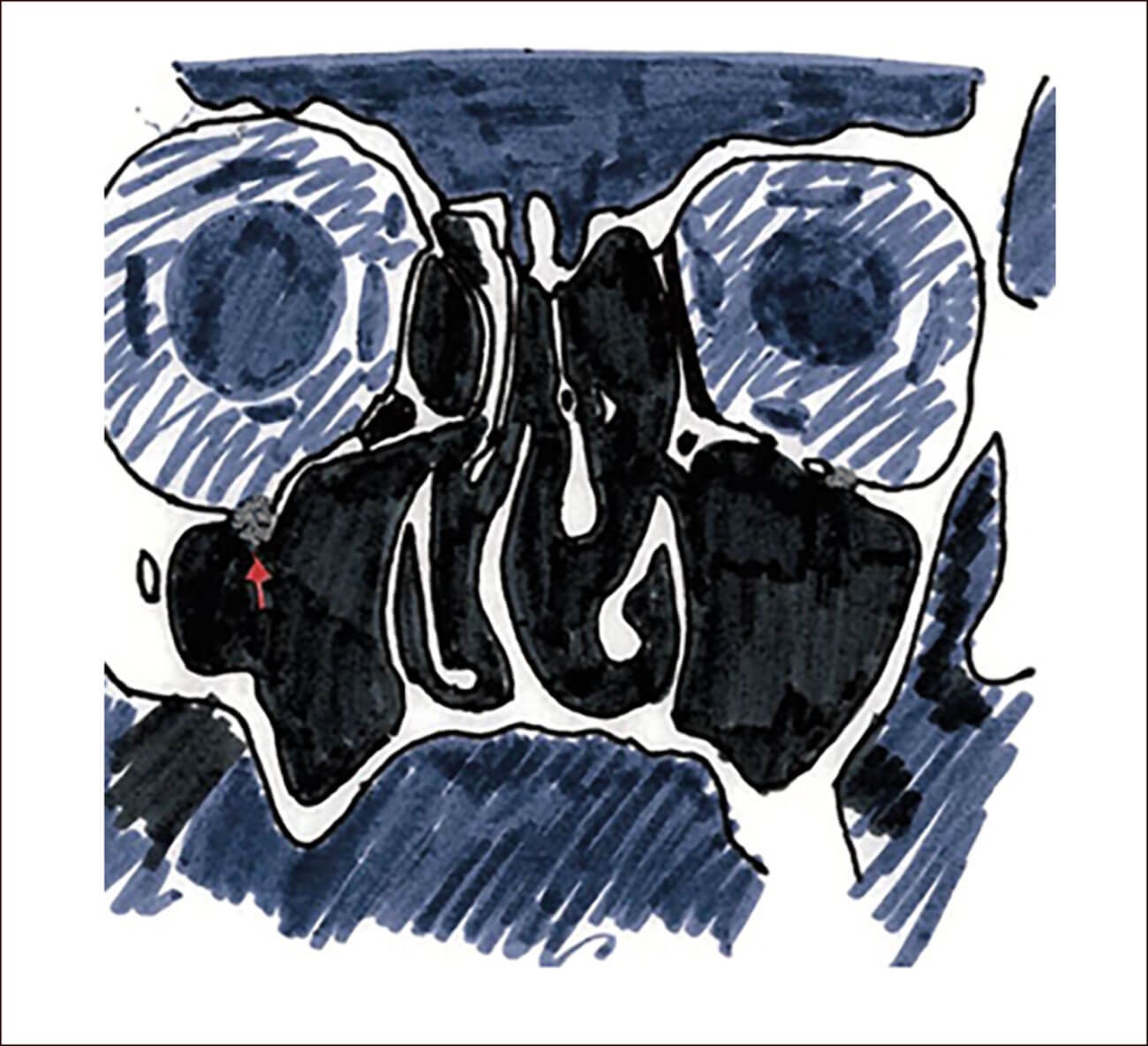
Image 1: Illustration of ION enlargement demonstrated
unilaterally on a computed tomography scan.
In current literature, up to 39% (25/65) of IgG4-ROD features trigeminal nerve involvement, approximately 64% of these are bilateral [2,8,14]. The common branch affected is the infraorbital nerve (ION), with accompanying enlargement of the infraorbital canal and even expansion into the infraorbital fissure [8]. The frontal nerve, supraorbital nerve and maxillary nerve have also been affected sparingly [8,10,20]. Very few entities cause enlargement of the ION and its canal, adnexal mucosa-associated lymphoid tissue (MALT) is another culprit, and notably this condition is linked to IgG4-ROD [21]. Image 1 is an illustration of ION enlargement demonstrated unilaterally on a computed tomography scan. Almost none other than IgG4-ROD will lead to bilateral ION enlargement [10]. This is a feature highly suggestive of IgG4-ROD. ION enlargement has been defined as larger than the optic nerve by some or two standard deviations above the mean by others [21,22]. Enlargement of the ION is also thought to be a predicting factor of extra-ophthalmic involvement [22,23]. A study of 68 patients found that 20 patients who had infraorbital nerve enlargement of >3.3mm were more likely to have extra-ophthalmic involvement than patients without infraorbital swelling, 29% versus 0% [22]. This change is rarely seen in isolation, usually accompanying other IgG4-ROD signs, enlargement of EOM particularly, lacrimal disease and paranasal sinus disease [10,23]. Although rare instances have been reported, involvement of nerves in IgG4-ROD does not often result in sensory loss [24,25]. Unusually, fibrosis is not usually a feature of IgG4 nerve involvement and the disease process appears to involve the epineurium mainly, alleviating fear of long-term implications [10,25].
In our study, 37.5% (3/8) of cases had IgG4 involvement of the EOM, in all involvement was unilateral. Reported frequencies of IgG4-ROD myositis are between 18% (40/225) to 89% (24/27) [2,8,13]. Of these, bilateral involvement is noted in up to 88% [2,8,13]. Single or multiple muscles could be affected [10]. Recent studies have found the lateral rectus most commonly affected and also to the greatest extent [13,26]. The inflammation in IgG4-ROD is tendon-sparing in up to 93-96% [8,13]. Active IgG4-ROD affecting the EOM usually allows better ocular motility, despite enlargement of these muscles, relative to differential inflammatory diseases such as thyroid eye disease and idiopathic orbital myositis [26-29, 30, 31]. Active episodes with increased serum IgG4 levels may produce a large-angle strabismus, but these mostly respond well to steroid treatment [26].
There has been suggestion that EOM / trigeminal nerve involvement in IgG4 ROD may signify a more progressive and recalcitrant disease course. In a large study, patients with EOM / trigeminal nerve enlargements required maintenance low-dose systemic corticosteroid treatment for stability, were more likely to be resistant to corticosteroid treatment and were significantly more likely to relapse after corticosteroid treatment [14]. This same study found a majority (18/20) of isolated lacrimal gland lesions who opted for watchful waiting, remained dormant or regressed spontaneously [14].
The optic nerve is sparingly affected in IgG4-ROD. The optic nerve sheath infiltration with IgG4 mass forming lesions near the orbital apex has been described, usually bilaterally and in one case associated with an extension to cause pachymeningitis [32-34]. As expected, common presentations include painless vision loss and non-specific visual fields defects. A focal optic nerve IgG4 lesion has been described and thought to represent a form of inflammatory demyelinating IgG4-driven disease, it was interesting to note, however, the absence of pain on presentation, which is a typical feature of inflammatory optic neuritis [35].
IgG4 orbital inflammation is thought to present in two forms: diffuse sclerosing orbital inflammation accounting for up to 23.1% (15/65) of IgG4-ROD, or demarcated orbital mass lesions in 16.9% (11/65); compression of the optic nerve causing visual symptoms and mass effect limiting globe movement are more common than IgG4 disease directly affecting the EOM and optic nerve [8]. In our findings, discrete orbital lesions were noted in 50% (4/8) and 12.5% (1/8) had diffuse orbital soft tissue involvement. However, as discussed, the figures in literature may be under-reported, thus a proportion of orbital inflammatory diagnoses previously thought to be idiopathic will now come under the IgG4-ROD definition [36]. While difficult to determine in retrospect, a review performed by Winn and Rootman found that a significant proportion (up to 50%) of orbital inflammation case reports had features suggestive of IgG4 disease [36].
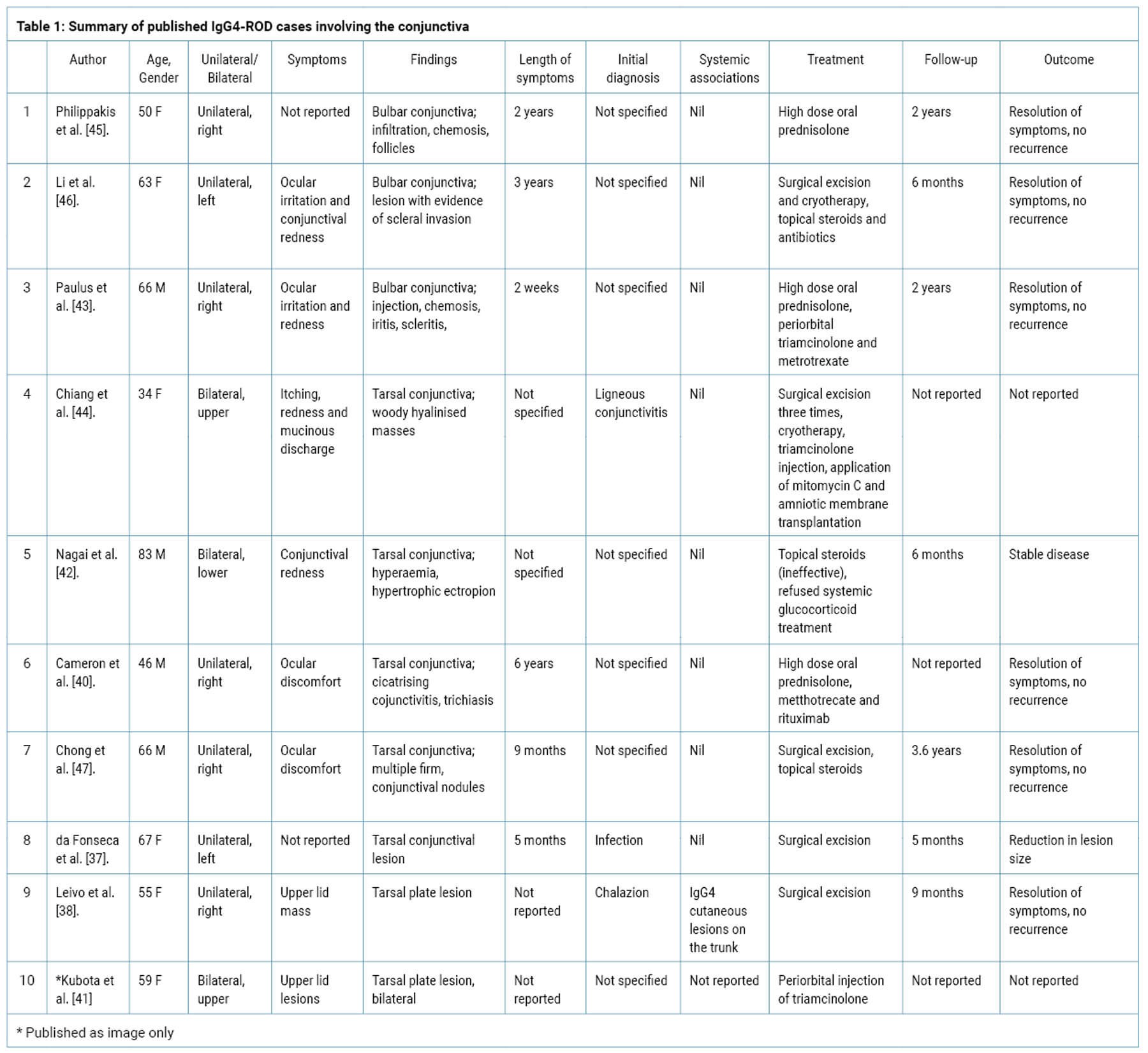
IgG4 involvement of the eyelid is uncommon and those affecting the tarso-conjunctival region are rare. Cutaneous eyelid lesions only account for about 12.3% of IgG4-ROD (8/65) and indeed the conjunctiva was previously thought to be a site not affected by IgG4 [5,8,15]. In recent years, only 10 cases involving the tarsal plate and conjunctiva have been confirmed and reported (Table 1) [37-47]. Interestingly, IgG4 disease in this region seems to vary in its presentation while other forms of IgG4-ROD are more uniform; these lesions have been misdiagnosed as chalazions, cicatrising or ligneous conjunctivitis or infection. Also unusually, the cases of IgG4-ROD presenting in this area were rarely associated with systemic IgG4 with the exception of one case who also had cutaneous trunk lesions (38). This has enabled several cases to be treated effectively with either pure local excision or local topical steroids; only three of the 10 cases requiring systemic treatment [37-46].
Other less commonly encountered IgG4-ROD includes ocular inflammatory forms; uveitis and scleritis [43,45,48,49]. IgG4 dacrocystitis is also infrequent; bilateral cases have been observed [8,50-53].
Treatment
Therapeutic decision-making in IgG4-ROD is often based on clinical experience and expert opinion due to the lack of level one evidence. Furthermore, because the molecular mechanisms sustaining IgG4-RD remain largely unknown, targeted therapies are not available. Active treatment may not always be necessary with careful observation appropriate for some asymptomatic cases, for example isolated lymphadenopathy or mild salivary gland enlargement and a large systematic review found this approach appropriate in 13% of the cohort, with spontaneous resolution [54,55].
Generally, however, IgG4 has a relapsing-remitting nature, and long-term immunosuppression is often needed. Risk factors for relapse include; elevated serum IgG4 at diagnosis, multi-organ involvement, previous relapse, and as discussed above, EOM / trigeminal nerve involvement [56].
Delivering a swift therapeutic response, generally within weeks, regardless of the clinical presentation and organ involvement, glucocorticoids is the central to treatment of IgG4-RD and is the current first-line treatment in patients with active disease [54,57]. With glucocorticoid monotherapy, a 97% therapeutic response was found in a cohort of 1220 patients in a systematic review [55]. Despite an overall good response to glucocorticoid, the recurrence of IgG4-ROD is not uncommon, after glucocorticoids are stopped or tapered off, with a relapse rate of up to 68.4% (17) [58-63]. Periorbital injection of glucocorticoids has been reported as an alternative treatment to systemic glucocorticoids approach for IgG4-ROD, particularly in the anterior orbit to avoid systemic immunosuppression [64]. Topical steroid use in eyelid / conjunctival lesions can be effective, easily applied with ease [39,65]. However repeated treatments may be needed in cases of relapse or incomplete response, which limits its use and increases the risk of local complication.
Immunomodulatory drugs are usually employed in cases of multiple relapses and where long-term maintenance is likely needed. A management consensus has advised that azathioprine, methotrexate or mycophenolate mofetil can be initiated as steroid-sparing agents in IgG4-RD (54) but other agents such as hydroxychloroquine, tacrolimus infliximab, thalidomide, cyclosporine have also been described as effective [54,61]. Within IgG4-ROD, a combination of glucocorticoids and immunomodulatory drugs (including cyclophosphamide, mycophenolate mofetil, methotrexate, leflunomide, azathioprine, iguratimod, and rituximab) has been found to be significantly protective against relapse when compared to glucocorticoid monotherapy alone; 43 months versus 17 months of relapse-free period [9].
The emergence of biological therapies has added to the IgG4-RD therapeutic armamentarium. Rituximab has already demonstrated promising results in refractory disease and has been used especially in IgG4-ROD cases to enable ‘medical’ orbital decompressions [2,66-69,70,71]. Nevertheless, relapse rates are still high once the therapy is ceased but has been used a successfully in maintenance, with a dosage every six months [67]. Rituximab also has the potential to be considered as first-line treatment, an open label trial found that two 100mg doses of rituximab resulted in a IgG4-RD response rate of 97%, largely (26 out of 30 patients) without glucocorticoid use and without re-administration of rituximab [72]. Yamamoto et al. have also reported the successful use of rituximab without glucocorticoids in IgG4-ROD dacryoadenitis [73].
There is also a role for surgery in managing this disease. There have been reports where excisional biopsies confirming IgG4 have inadvertently functioned as effective treatment [73-75]. An example within IgG4-ROD; enucleation where the initial suspicion was a choroidal tumour presenting as a white subretinal mass, IgG4 disease was later diagnosed and serum IgG4 levels reduced after excision without any other treatment [48]. Subsequently, primary surgical resection of IgG4-related tissues has been described in the thyroid gland, pulmonary tissue, pericardium and pancreas leading to prolonged periods of remission (median 36 months), in some cases without further immunosuppressive therapy [61]. This therefore implies that resection of the lesions and debulking of the target organs involved in the disease may result in a lower recurrence rate than steroid therapy, where the target organs are preserved [61]. There has been some experience to variable success in surgical debulking or excision as primary treatment for IgG4-ROD affecting eyelid lesions, the lacrimal gland, nasolacrimal duct and orbital fat [2,5,76]. Otherwise, IgG4-ROD rarely necessitates surgical treatment, except for sight orbital decompression or enucleation as a last measure for symptom relief [77,78].
Conclusion
IgG4 can strike almost any ocular region, with varying presentations and with multiple areas affected simultaneously, creating a complicated clinical picture. However, it is an important diagnosis to consider and establish, as lack of treatment may allow the disease to progress unchecked and result in devastating outcomes such as loss of vision, the eye itself and ocular adnexa. Furthermore, there are effective treatments for IgG-ROD with good response rates, although the long-term management of relapses is still an issue.
TAKE HOME MESSAGES
-
IgG4 disease can affect nearly any ocular and adnexal structure. Multiple areas can be affected simultaneously or synchronously and present variably. Therefore, IgG4-ROD should be considered in presentations of ocular and especially orbital inflammation
-
Risk factors for relapses include: EOM / trigeminal nerve involvement, multi-organ involvement, previous relapses plus raised serum IgG4 and male gender (as discussed in Part 1).
-
Currently, there are several effective lines of treatment for IgG4-ROD though long-term management of relapses is still an issue.
References
1. Stone JH, Khosroshahi A, Deshpande V, Chan JK, Heathcote JG, Aalberse R, et al. Recommendations for the nomenclature of IgG4-related disease and its individual organ system manifestations. Arthritis Rheum. 2012;64(10):3061-7.
2. Wallace ZS, Deshpande V, Stone JH. Ophthalmic manifestations of IgG4-related disease: single-center experience and literature review. Semin Arthritis Rheum. 2014;43(6):806-17.
3. Cheuk W, Yuen HK, Chan JK. Chronic sclerosing dacryoadenitis: part of the spectrum of IgG4-related Sclerosing disease? Am J Surg Pathol. 2007;31(4):643-5.
4. Matsuo T, Ichimura K, Sato Y, Tanimoto Y, Kiura K, Kanazawa S, et al. Immunoglobulin G4 (IgG4)-positive or -negative ocular adnexal benign lymphoid lesions in relation to systemic involvement. J Clin Exp Hematop. 2010;50(2):129-42.
5. Sato Y, Ohshima K, Ichimura K, Sato M, Yamadori I, Tanaka T, et al. Ocular adnexal IgG4-related disease has uniform clinicopathology. Pathol Int. 2008;58(8):465-70.
6. Plaza JA, Garrity JA, Dogan A, Ananthamurthy A, Witzig TE, Salomão DR. Orbital inflammation with IgG4-positive plasma cells: manifestation of IgG4 systemic disease. Arch Ophthalmol. 2011;129(4):421-8.
7. Go H, Kim JE, Kim YA, Chung HK, Khwarg SI, Kim CW, et al. Ocular adnexal IgG4-related disease: comparative analysis with mucosa-associated lymphoid tissue lymphoma and other chronic inflammatory conditions. Histopathology. 2012;60(2):296-312.
8. Sogabe Y, Ohshima K, Azumi A, Takahira M, Kase S, Tsuji H, et al. Location and frequency of lesions in patients with IgG4-related ophthalmic diseases. Graefes Arch Clin Exp Ophthalmol. 2014;252(3):531-8.
9. Zhao Z, Mou D, Wang Z, Zeng Q, Xue J, Ren L, et al. Clinical features and relapse risks of IgG4-related ophthalmic disease: a single-center experience in China. Arthritis Res Ther. 2021;23(1):98.
10. McNab AA, McKelvie P. IgG4-Related Ophthalmic Disease. Part II: Clinical Aspects. Ophthalmic Plast Reconstr Surg. 2015;31(3):167-78.
11. Koizumi S, Kamisawa T, Kuruma S, Tabata T, Iwasaki S, Chiba K, et al. Clinical features of IgG4-related dacryoadenitis. Graefes Arch Clin Exp Ophthalmol. 2014;252(3):491-7.
12. Hagiya C, Tsuboi H, Yokosawa M, Hagiwara S, Hirota T, Takai C, et al. Clinicopathological features of IgG4-related disease complicated with orbital involvement. Mod Rheumatol. 2014;24(3):471-6.
13. Tiegs-Heiden CA, Eckel LJ, Hunt CH, Diehn FE, Schwartz KM, Kallmes DF, et al. Immunoglobulin G4-related disease of the orbit: imaging features in 27 patients. AJNR Am J Neuroradiol. 2014;35(7):1393-7.
14. Kubota T, Katayama M, Nishimura R, Moritani S. Long-term outcomes of ocular adnexal lesions in IgG4-related ophthalmic disease. Br J Ophthalmol. 2020;104(3):345-9.
15. Andrew N, Kearney D, Selva D. IgG4-related orbital disease: a meta-analysis and review. Acta Ophthalmol. 2013;91(8):694-700.
16. Kubota T, Moritani S, Katayama M, Terasaki H. Ocular adnexal IgG4-related lymphoplasmacytic infiltrative disorder. Arch Ophthalmol. 2010;128(5):577-84.
17. Yu WK, Kao SC, Yang CF, Lee FL, Tsai CC. Ocular adnexal IgG4-related disease: clinical features, outcome, and factors associated with response to systemic steroids. Jpn J Ophthalmol. 2015;59(1):8-13.
18. Takahira M, Kawano M, Zen Y, Minato H, Yamada K, Sugiyama K. IgG4-Related Chronic Sclerosing Dacryoadenitis. Arch Ophthalmol. 2007;125(11):1575-8.
19. Wu A, Andrew NH, McNab AA, Selva D. Bilateral IgG4-related ophthalmic disease: a strong indication for systemic imaging. Br J Ophthalmol. 2016;100(10):1409-11.
20. Toyoda K, Oba H, Kutomi K, Furui S, Oohara A, Mori H, et al. MR imaging of IgG4-related disease in the head and neck and brain. AJNR Am J Neuroradiol. 2012;33(11):2136-9.
21. Ohshima K, Sogabe Y, Sato Y. The usefulness of infraorbital nerve enlargement on MRI imaging in clinical diagnosis of IgG4-related orbital disease. Jpn J Ophthalmol. 2012;56(4):380-2.
22. Takano K, Yajima R, Seki N, Abe A, Yamamoto M, Takahashi H, et al. A study of infraorbital nerve swelling associated with immunoglobulin G4 Mikulicz’s disease. Mod Rheumatol. 2014;24(5):798-801.
23. Hardy TG, McNab AA, Rose GE. Enlargement of the infraorbital nerve: an important sign associated with orbital reactive lymphoid hyperplasia or immunoglobulin g4-related disease. Ophthalmology. 2014;121(6):1297-303.
24. Katsura M, Mori H, Kunimatsu A, Sasaki H, Abe O, Machida T, et al. Radiological features of IgG4-related disease in the head, neck, and brain. Neuroradiology. 2012;54(8):873-82.
25. Sogabe Y, Miyatani K, Goto R, Ishii G, Ohshima K, Sato Y. Pathological findings of infraorbital nerve enlargement in IgG4-related ophthalmic disease. Jpn J Ophthalmol. 2012;56(5):511-4.
26. Kim N, Yang HK, Kim JH, Hwang JM. IgG4-related ophthalmic disease involving extraocular muscles: case series. BMC Ophthalmol. 2018;18(1):162.
27. Shin YU, Oh YH, Lee YJ. Unusual involvement of IgG4-related sclerosing disease in lacrimal and submandibular glands and extraocular muscles. Korean J Ophthalmol. 2012;26(3):216-21.
28. Wallace ZS, Khosroshahi A, Jakobiec FA, Deshpande V, Hatton MP, Ritter J, et al. IgG4-related systemic disease as a cause of “idiopathic” orbital inflammation, including orbital myositis, and trigeminal nerve involvement. Surv Ophthalmol. 2012;57(1):26-33.
29. Higashiyama T, Nishida Y, Ugi S, Ishida M, Nishio Y, Ohji M. A case of extraocular muscle swelling due to IgG4-related sclerosing disease. Jpn J Ophthalmol. 2011;55(3):315-7.
30. Kubota T, Kano H. Assessment of inflammation in idiopathic orbital myositis with fat-suppressed T2-weighted magnetic resonance imaging. Am J Ophthalmol. 2007;143(4):718-20.
31. Bartley GB, Fatourechi V, Kadrmas EF, Jacobsen SJ, Ilstrup DM, Garrity JA, et al. Clinical features of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121(3):284-90.
32. Ramirez L, D’Auria A, Popalzai A, Sanossian N. Bilateral Vision Loss Secondary to Pachymeningitis in a Patient with IgG4-Related Disease. Front Neurol. 2014;5:192.
33. Takahira M, Ozawa Y, Kawano M, Zen Y, Hamaoka S, Yamada K, et al. Clinical Aspects of IgG4-Related Orbital Inflammation in a Case Series of Ocular Adnexal Lymphoproliferative Disorders. Int J Rheumatol. 2012;2012:635473.
34. Takahashi Y, Kitamura A, Kakizaki H. Bilateral optic nerve involvement in immunoglobulin G4-related ophthalmic disease. J Neuroophthalmol. 2014;34(1):16-9.
35. Zhang W, Luo J, Jiao J. Optic nerve involvement in immunoglobulin G4-related disease: A case report. Exp Ther Med. 2016;12(1):111-4.
36. Winn BJ, Rootman J. Sclerosing orbital inflammation and systemic disease. Ophthalmic Plast Reconstr Surg. 2012;28(2):107-18.
37. da Fonseca FL, Ramos ReI, de Lima PP, Nogueira AB, Matayoshi S. Unilateral eyelid mass as an unusual presentation of ocular adnexal IgG4-related inflammation. Cornea. 2013;32(4):517-9.
38. Leivo T, Koskenmies S, Uusitalo M, Tynninen O. IgG4-related disease mimicking chalazion in the upper eyelid with skin manifestations on the trunk. Int Ophthalmol. 2015;35(4):595-7.
39. SSY C. A Case of IgG4-Related Sclerosing Disease Presenting at the Tarsal Conjunctiva. Journal of Clinical & Experimental Ophthalmology. 2018;9(1):1-3.
40. Cameron CA, Juniat V, Mills RAD, Hughes T, Klebe S, Selva D. IgG4-related Disease Presenting as Isolated Cicatrising Conjunctivitis. Ophthalmic Plast Reconstr Surg. 2021;37(3):e114-e7.
41. Kubota T, Moritani S, Sakuma M. Tarsal IgG4-related disease. JAMA Ophthalmol. 2015;133(2):e143272.
42. Nagai T, Yunoki T, Hayashi A. A Case of IgG4-Related Bilateral Palpebral Conjunctivitis. Case Rep Ophthalmol. 2019;10(2):299-303.
43. Paulus YM, Cockerham KP, Cockerham GC, Gratzinger D. IgG4-positive sclerosing orbital inflammation involving the conjunctiva: a case report. Ocul Immunol Inflamm. 2012;20(5):375-7.
44. Chiang WY, Liu TT, Huang WT, Kuo MT. Co-existing ligneous conjunctivitis and IgG4-related disease. Indian J Ophthalmol. 2016;64(7):532-4.
45. Philippakis E, Cassoux N, Charlotte F, LeHoang P, Bodaghi B, Bloch-Queyrat C, et al. IgG4-related Disease Masquerading as Recurrent Scleritis and Chronic Conjunctivitis. Ocul Immunol Inflamm. 2015;23(2):168-72.
46. Li A, Plesec TP, Mileti L, Singh AD. Isolated Conjunctival Inflammation as a Manifestation of IgG4-Related Disease. Cornea. 2018;37(9):1182-4.
47. Chong et al. A Case of IgG4-Related Sclerosing Disease Presenting at the Tarsal Conjunctiva. Journal of Clinical & Experimental Ophthalmology. 2018;9(1):1-3.
48. Ohno K, Sato Y, Ohshima K, Takata K, Ando M, Abd Al-Kader L, et al. IgG4-related disease involving the sclera. Mod Rheumatol. 2014;24(1):195-8.
49. Kim EC, Lee SJ, Hwang HS, Kim J, Kim MS. Bilateral diffuse scleritis as a first manifestation of immunoglobulin G4-related sclerosing pachymeningitis. Can J Ophthalmol. 2013;48(2):e31-3.
50. Kase S, Suzuki Y, Shinohara T, Kase M. IgG4-related lacrimal sac diverticulitis. Orbit. 2014;33(3):217-9.
51. Ginat DT, Freitag SK, Kieff D, Grove A, Fay A, Cunnane M, et al. Radiographic patterns of orbital involvement in IgG4-related disease. Ophthalmic Plast Reconstr Surg. 2013;29(4):261-6.
52. Suzuki M, Mizumachi T, Morita S, Kubota K, Iizuka K. A case of immunoglobulin 4-related disease with bilateral mass-forming lesions in the nasolacrimal ducts. J Clin Rheumatol. 2011;17(4):207-10.
53. Batra R, Mudhar HS, Sandramouli S. A unique case of IgG4 sclerosing dacryocystitis. Ophthalmic Plast Reconstr Surg. 2012;28(3):e70-2.
54. Khosroshahi A, Wallace ZS, Crowe JL, Akamizu T, Azumi A, Carruthers MN, et al. International Consensus Guidance Statement on the Management and Treatment of IgG4-Related Disease. Arthritis Rheumatol. 2015;67(7):1688-99.
55. Brito-Zerón P, Kostov B, Bosch X, Acar-Denizli N, Ramos-Casals M, Stone JH. Therapeutic approach to IgG4-related disease: A systematic review. Medicine (Baltimore). 2016;95(26):e4002.
56. Bledsoe JR, Della-Torre E, Rovati L, Deshpande V. IgG4-related disease: review of the histopathologic features, differential diagnosis, and therapeutic approach. APMIS. 2018;126(6):459-76.
57. Yu WK, Tsai CC, Kao SC, Liu CJ. Immunoglobulin G4-related ophthalmic disease. Taiwan J Ophthalmol. 2018;8(1):9-14.
58. Campochiaro C, Ramirez GA, Bozzolo EP, Lanzillotta M, Berti A, Baldissera E, et al. IgG4-related disease in Italy: clinical features and outcomes of a large cohort of patients. Scand J Rheumatol. 2016;45(2):135-45.
59. Derzko-Dzulynsky L. IgG4-related disease in the eye and ocular adnexa. Curr Opin Ophthalmol. 2017;28(6):617-22.
60. Park J, Lee MJ, Kim N, Kim JE, Park SW, Choung HK, et al. Risk factors for extraophthalmic involvement and treatment outcomes in patients with IgG4-related ophthalmic disease. Br J Ophthalmol. 2018;102(6):736-41.
61. Karim AF, Bansie RD, Rombach SM, Paridaens D, Verdijk RM, van Hagen PM, et al. The treatment outcomes in IgG4-related disease. Neth J Med. 2018;76(6):275-85.
62. Suimon Y, Kase S, Ishijima K, Kanno-Okada H, Ishida S. A clinicopathological study on IgG4-related ophthalmic disease. Int J Ophthalmol. 2018;11(9):1539-44.
63. Ebbo M, Patient M, Grados A, Groh M, Desblaches J, Hachulla E, et al. Ophthalmic manifestations in IgG4-related disease: Clinical presentation and response to treatment in a French case-series. Medicine (Baltimore). 2017;96(10):e6205.
64. Andrew NH, Gajdatsy A, Selva D. Intraorbital corticosteroid injection for the treatment of IgG4-related ophthalmic disease. Br J Ophthalmol. 2016;100(5):644-7.
65. Verhoekx JSN, Karim AF, van Laar JAM, Verdijk RM, Paridaens D. The tarsal plate manifestation of IgG4-related disease. Int Ophthalmol. 2019;39(7):1613-5.
66. Brito-Zerón P, Bosch X, Ramos-Casals M, Stone JH. IgG4-related disease: Advances in the diagnosis and treatment. Best Pract Res Clin Rheumatol. 2016;30(2):261-78.
67. Campochiaro C, Della-Torre E, Lanzillotta M, Bozzolo E, Baldissera E, Milani R, et al. Long-term efficacy of maintenance therapy with Rituximab for IgG4-related disease. Eur J Intern Med. 2020;74:92-8.
68. Khosroshahi A, Carruthers MN, Deshpande V, Unizony S, Bloch DB, Stone JH. Rituximab for the treatment of IgG4-related disease: lessons from 10 consecutive patients. Medicine (Baltimore). 2012;91(1):57-66.
69. Khosroshahi A, Bloch DB, Deshpande V, Stone JH. Rituximab therapy leads to rapid decline of serum IgG4 levels and prompt clinical improvement in IgG4-related systemic disease. Arthritis Rheum. 2010;62(6):1755-62.
70. Chen TS, Figueira E, Lau OC, McKelvie PA, Smee RI, Dawes LC, et al. Successful “medical” orbital decompression with adjunctive rituximab for severe visual loss in IgG4-related orbital inflammatory disease with orbital myositis. Ophthalmic Plast Reconstr Surg. 2014;30(5):e122-5.
71. Wu A, Andrew NH, Tsirbas A, Tan P, Gajdatsy A, Selva D. Rituximab for the treatment of IgG4-related orbital disease: experience from five cases. Eye (Lond). 2015;29(1):122-8.
72. Carruthers MN, Topazian MD, Khosroshahi A, Witzig TE, Wallace ZS, Hart PA, et al. Rituximab for IgG4-related disease: a prospective, open-label trial. Ann Rheum Dis. 2015;74(6):1171-7.
73. Yamamoto M, Awakawa T, Takahashi H. Is rituximab effective for IgG4-related disease in the long term? Experience of cases treated with rituximab for 4 years. Ann Rheum Dis. 2015;74(8):e46.
74. Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, et al. Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology. 2008;134(3):706-15.
75. Zen Y, Inoue D, Kitao A, Onodera M, Abo H, Miyayama S, et al. IgG4-related lung and pleural disease: a clinicopathologic study of 21 cases. Am J Surg Pathol. 2009;33(12):1886-93.
76. Raissian Y, Nasr SH, Larsen CP, Colvin RB, Smyrk TC, Takahashi N, et al. Diagnosis of IgG4-related tubulointerstitial nephritis. J Am Soc Nephrol. 2011;22(7):1343-52.
77. Ominato J, Oyama T, Cho H, Shiozaki N, Umezu H, Takizawa J, et al. The natural course of IgG4-related ophthalmic disease after debulking surgery: a single-centre retrospective study. BMJ Open Ophthalmol. 2019;4(1):e000295.
78. al. NRe. Surgical management for IgG4-related ophthalmic disease by a transcranial biopsy combined with extraorbital decompression: illustrative case. J Neurosurg Case Lessons. 2021;1(8):1-5.
79. Lee CS, Harocopos GJ, Kraus CL, Lee AY, Van Stavern GP, Couch SM, et al. IgG4-associated orbital and ocular inflammation. J Ophthalmic Inflamm Infect. 2015;5:15.
COMMENTS ARE WELCOME