It has been a few years now since we started these surveys and I continue to be amazed by the variance in our practice. As a patient, I think I would expect there to be more consistency and evidence-based practice. This is not meant as a criticism but highlights the need for us to recognise, discuss and act on variance to do the best for our patients.
Ophthalmology is an art to some degree and there will be practice variance which should be encouraged, as that is how our specialty evolves and improves. If there is good practice variance then we need to ensure there are avenues to educate others and reach the ‘coal face’ of ophthalmology with the information.
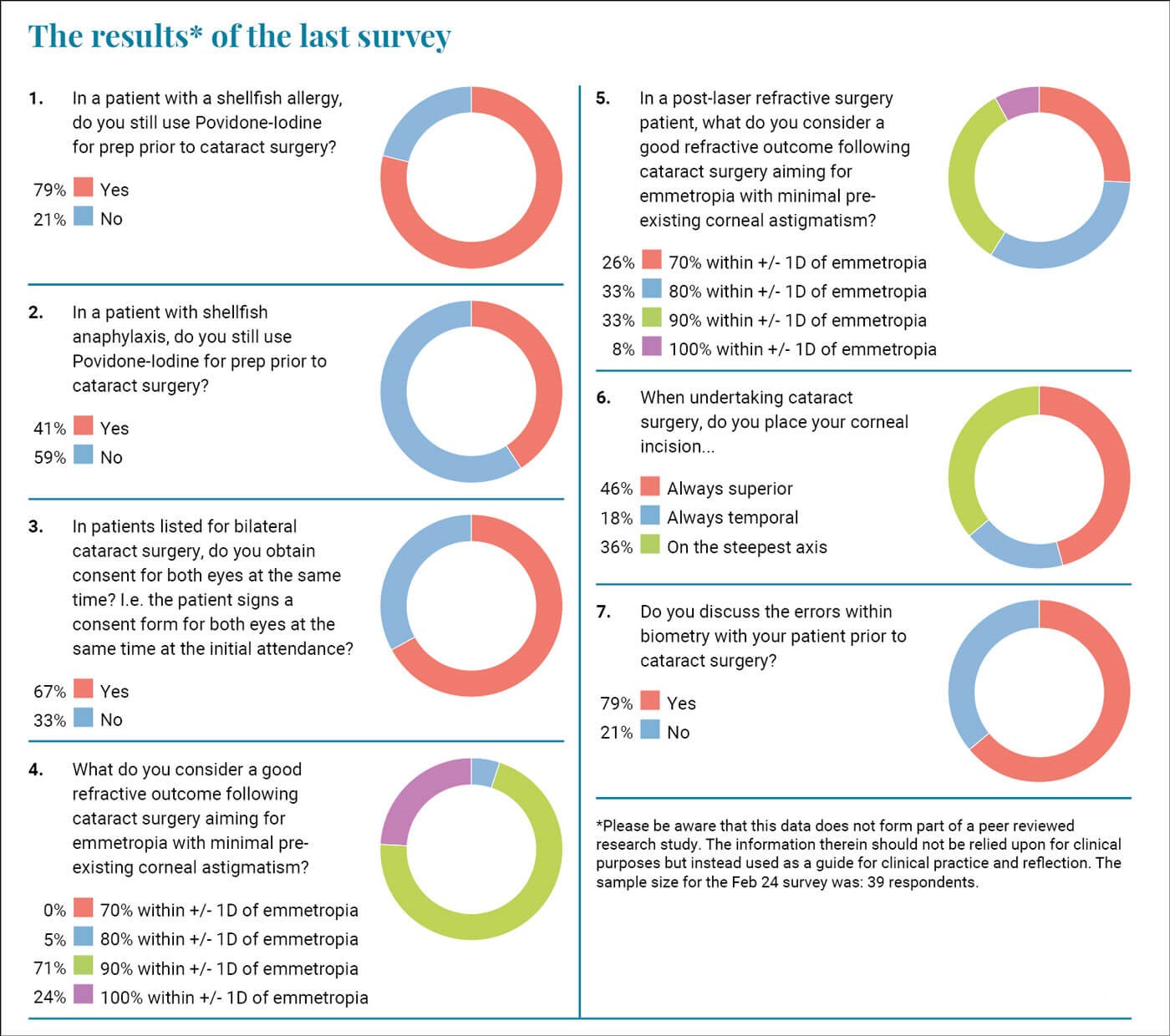
The first two questions refer to a commonly asked query regarding shell-fish allergy and the use of Povidone-iodine (PI). I still get surgical teams looking at me sceptically when I suggest we will continue to use my usual prep in a patient with shell-fish allergy. I seem to remember this being an issue during my training a few (ahem…) years ago.
Povidone-iodine (Betadine) is a chemical complex of a water-soluble polymer povidone and triiodide. The antimicrobial action of iodine occurs after it dissociates from the complex. Free iodine rapidly penetrates microbial cell membranes and interacts with proteins, nucleotides, and fatty acids in the cytoplasm and cytoplasmic membranes, resulting in rapid cell death within 15 seconds [1]. Povidone-iodine is bactericidal, fungicidal, and virucidal. Resistance to PI has not been reported so it is really good stuff for preoperative preparation on any surgical site. It is widely used around and on the ocular surface prior to ocular surgery.
Current US guidelines recommend administration of 5–10% PI at least 30 seconds prior to intravitreal injections [1]. It can be safely used in most patients with self-reported iodine or shellfish allergy [2]. This is because the so-called allergy to iodinated contrast agents is a reaction to hyperosmolar solutions, and allergy to shellfish is due to tropomyosin, a muscle protein, and not to iodine, a vital micronutrient [3].
Even a food allergy website for patients makes this clear; “If you have a shellfish allergy, you do not need to worry about cross-reactions with iodine or radiocontrast material (which can contain iodine and is used in some radiographic medical procedures)” [4].
Povidone-iodine prep works and there is evidence of increasing risks of infection when it is omitted. Therefore, I am concerned that 21% of you do not use PI when there is a history of shellfish allergy, and this increases up to 69% when there is shellfish-related anaphylaxis. The evidence is not there for this practice and if a patient does develop an infection, can we confidently say that we did our best to minimise the risk notwithstanding the fact that clearly another agent will be used?
We have previously discussed immediately sequential bilateral cataract surgery and informed consent is clearly vital for that as there is a miniscule but still material risk of bilateral issues. This question refers to patients having one eye done and then the other done at a later date. Two-thirds of you are happy to consent for both eyes at the first attendance. I have no objection to this as that is the time when we have the chance to explain everything and answer the patient’s questions. Often patients are listed for their second eye without a further review, so this seems logical practice to do it at the primary cataract clinic attendance. It is vital though that the consent is confirmed on the day of surgery, and it is made clear to the patient that the risks of surgery are the same as the first-time round. Patients would have had their first eye done and it is likely it would have been smooth without any issues. They reasonably assume that there will be no issues with their second eye, and indeed that is the likelihood but not the certainty. They need to be fully informed that just because the first eye encountered no issue there is no guarantee that the second eye will not run into complications. From a self-preservation perspective it is vital that patients know this as if things do go wrong with their second eye and someone else did the first eye, they will commonly start to consider whether they were mismanaged for their second procedure and start questioning the competence of their second eye surgeon.
The next question relates to visual outcomes. The biggest, and thankfully only, real source of dissatisfaction amongst my surgical patients (I primarily undertake cataract and refractive surgery) is suboptimal (“not perfect”) distance vision. Often, I am let down by the biometry and I have also been surprised by an unexpected cylinder that materialises postoperatively. I try and make it clear to patients that there is no guarantee and I routinely discuss the biometry errors and lack of 100% predictability with them. I give them an information leaflet that explains it all in depth and yet still I get patients who are unhappy and talk about the “wrong” lens being put in.
A quarter of you felt that in non-laser refractive surgery (LRS) patients should achieve an outcome within +/- 1D of emmetropia all of the time. The rest of you allowed a slightly larger margin of error with 71% saying that 90% should achieve this threshold and 5% saying that we should be happy achieving this 80% of the time. When facing the same question with a post-LRS patient there was, correctly, a larger accepted margin of error. Refractive surprises can occur in these patients and despite my best efforts I see them. Warning the patient is vital and consent is key. These are visually demanding patients who had previous LRS because they are visually demanding. Sharing the results of this survey with them may help them understand that we cannot guarantee outcomes.
I was reflecting on the outcome of question four and five and tried to correlate this with outcomes of other forms of surgery. I could not think of any other branch of surgery where patients could readily determine what they would have been like had a different strength / size / power of implant was used. If a patient has a total hip replacement with a 32mm implant head and still experiences stiffness and a slight bit of nighttime pain, then there is no way for them to know whether this would not have been the case if they had had a 34mm implant instead. Whereas for us our patients can have a completely and technically successful procedure, and they attend their optometrist achieving 6/6 unaided but 6/4 with a -0.50/-0.5D refraction. They immediately question why they cannot achieve their ‘perfect’ vision and the answer is that if you had implanted a 23.5D IOL instead of a 24D and placed your corneal incision a bit higher then may indeed have achieved that.
I think it is important to inform patients of this and particularly so if they are having their procedure in the private sector. Jumping ahead one question, 79% of you do inform your patients which I think is good practice.
Regarding the question on your corneal incision practice we find a significant split in practice. Almost half of you routinely place your incision superiorly, while 18% always place it temporally, and finally one third place it on the steepest axis. As mentioned above residual cylinders are often a source of dissatisfaction in my patients so I try to place my incision on the steepest axis. This is not always possible and can be subject to logistic issues. For example, at one of my practices the orientation of the operating theatre makes it hard to undertake a temporal approach. In general, I think that trying to place the incision on the steepest axis of the cornea makes sense.
As ever I am grateful for your assistance with the survey and your time reading this. I hope it will give you food for thought and assist you in reflecting on your own practice.
References
1. Avery RL, Bakri SJ, Blumenkranz MS, et al. Intravitreal injection technique and monitoring: updated guidelines of an expert panel. Retina 2014;34(Suppl 12):S1–18.
2. Wykoff CC, Flynn HW, Han DP. Allergy to povidone-iodine and cephalosporins: the clinical dilemma in ophthalmic use. Am J Ophthalmol 2011;151(1):4–6.
3. Stewart MW. Doctor I have an iodine allergy. Ophthalmol Ther 2022;11(3):931–8.
4. Shellfish allergy. Food Allergy Research & Education.
https://www.foodallergy.org/living-food
-allergies/food-allergy-essentials/
common-allergens/shellfish
[Link last accessed March 2024]
COMMENTS ARE WELCOME