Thank you to all those who participated in this edition’s survey and for those of you who attended my recent Medico-legal Seminar at the Royal College of Ophthalmologists. It was a fascinating and educational day, made a success by the enthusiasm and engagement of the delegates. This next survey addresses some common ‘bread and butter’ issues we face on a daily basis.
Cystoid macular oedema (CMO) is a common complication of apparently routine cataract surgery, with published incidence rates ranging from 1.2–3.4% [1-5]. Known as Irvine-Gass syndrome, patients commonly experience good vision in the immediate postoperative period, followed by a painless central visual deterioration a few weeks later [3]. CMO is believed to result from a postoperative inflammatory response [6]. The incidence of postoperative CMO peaks at 4–6 weeks, but most cases are self-limiting, though visual impairment can persist, with duration of CMO reported to range between 72 and 249 days [7].
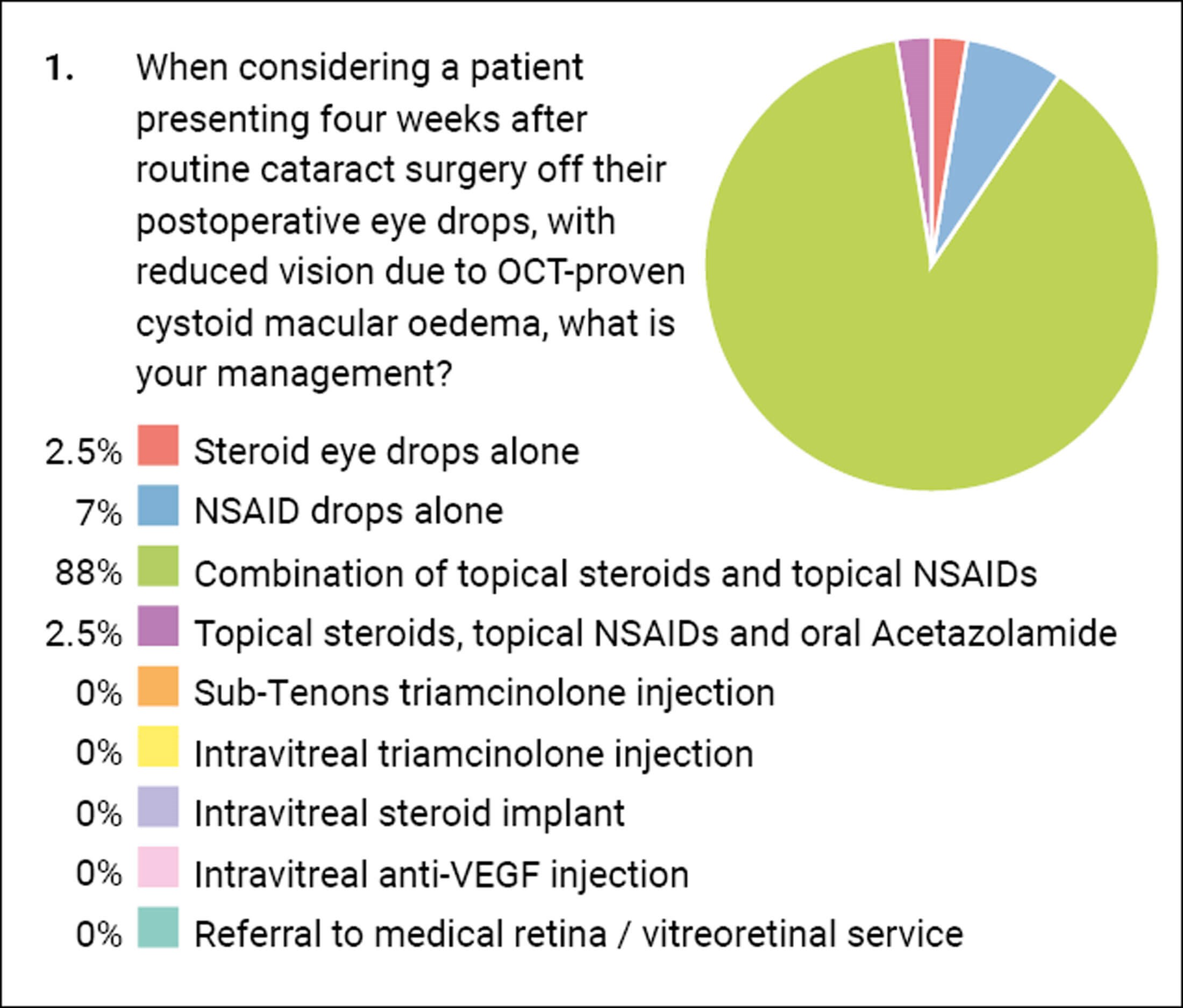
Our first question relates to the management of CMO presenting four weeks postoperatively. There appears to be a consensus that treatment should be with a combination of topical steroid and non-steroidal anti-inflammatory drugs (NSAID) with 88% of you agreeing. A small proportion of you use only a steroid or an NSAID, however in my personal opinion both are required ideally. The use of acetazolamide is often not without its risks to elderly patients with co-morbidity. The literature does have some evidence for its use in certain chronic inflammatory diseases, but I think it’s probably overkill for ‘simple’ postoperative CMO.
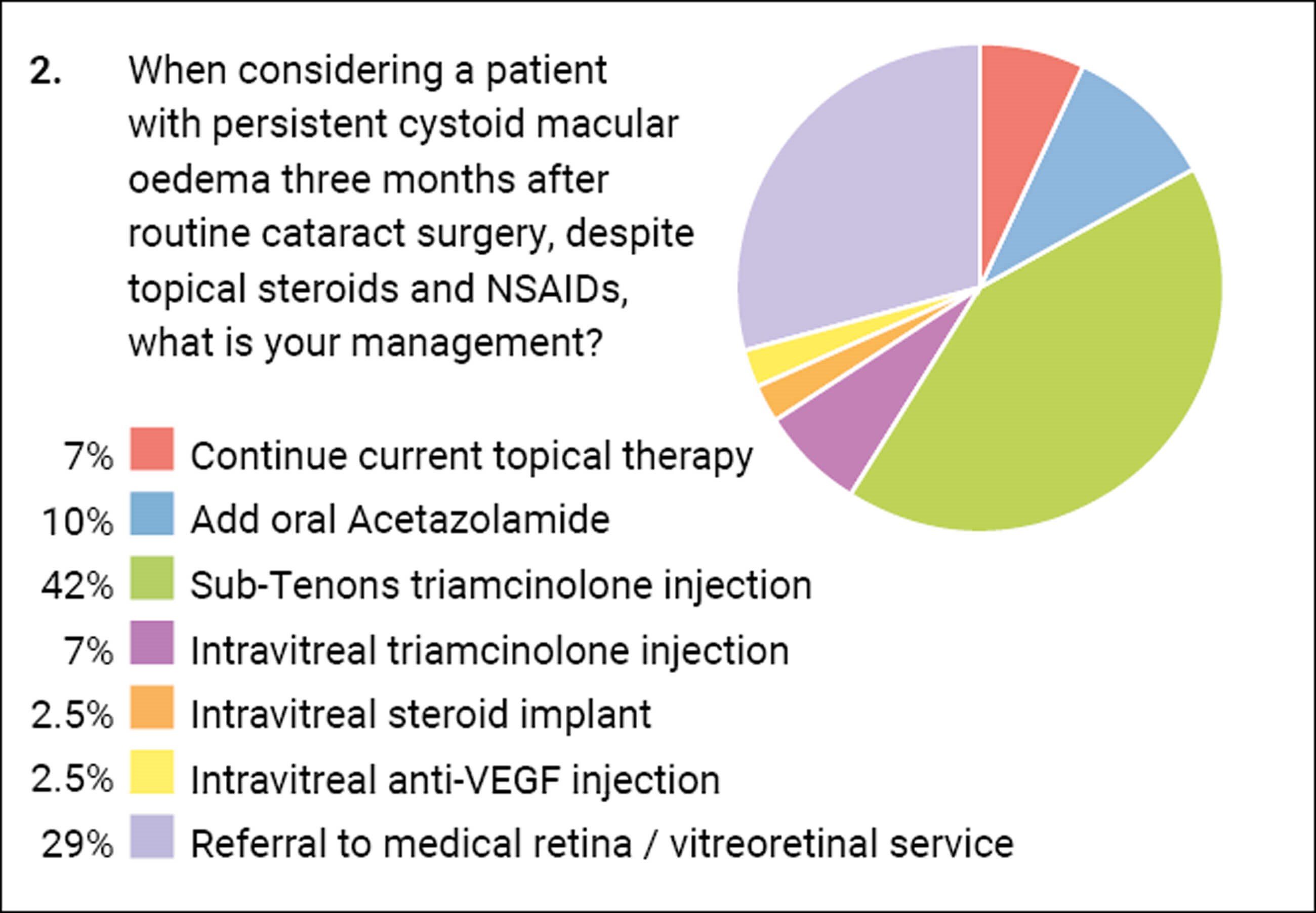
The next question deals with the patient who has failed to settle. Almost one third would refer onto another colleague for assistance. Almost half would give a depot periocular steroid injection which is also my favoured next step at this stage. Others are giving intravitreal steroid or anti-VEGF agents.
Question 3 takes the clinical scenario a step further with CMO now persisting to six months postoperatively, a frustrating situation for both surgeon and patient – now almost half of us would seek assistance. There is a spread of other treatments, with a fifth of us electing for an intravitreal steroid implant such as Ozurdex™.
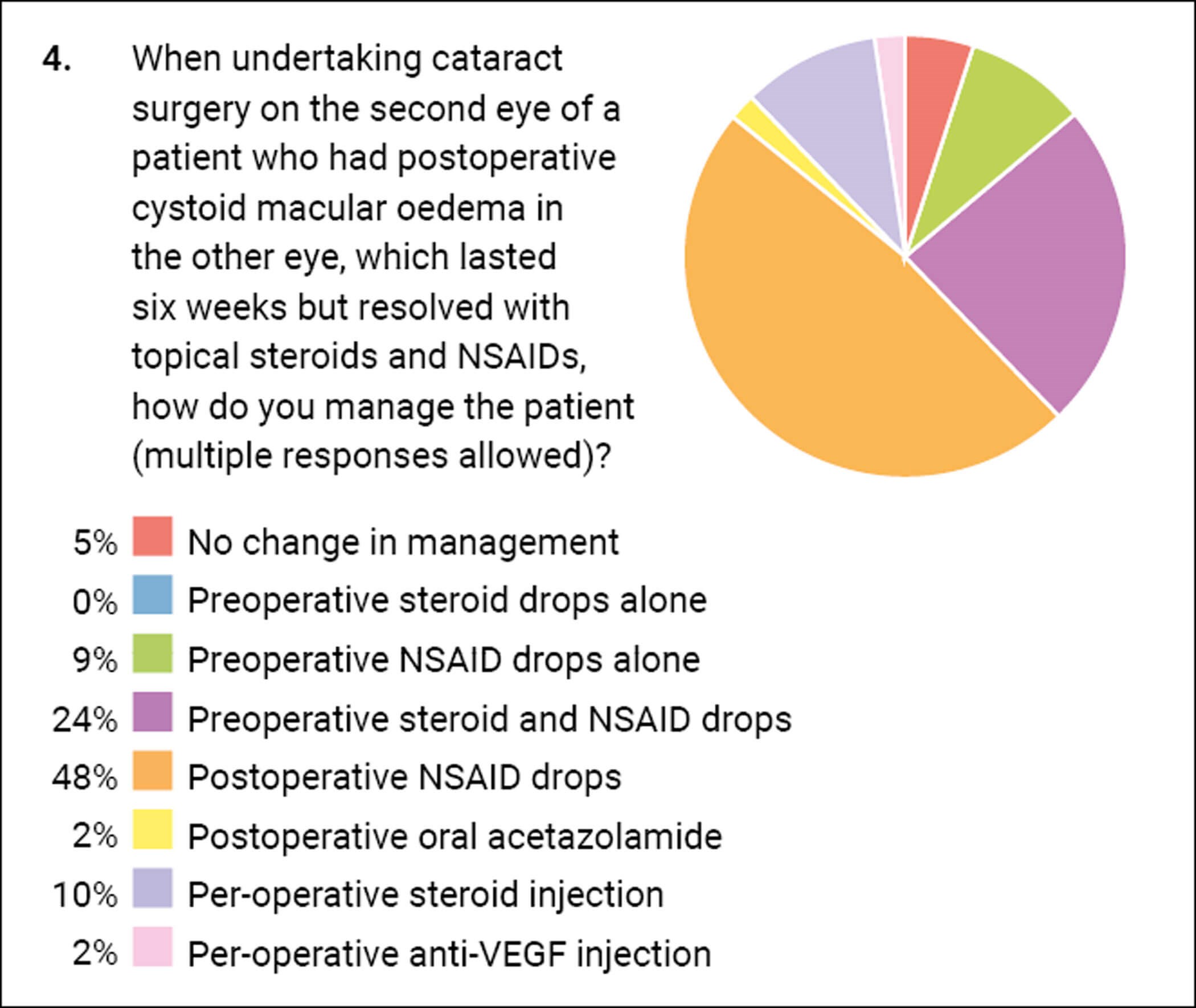
What is clear from the responses is that there is a variance of opinion regarding management, and thus guidance would be welcome. When undertaking second eye surgery on a patient who has had CMO in their first eye, the majority of you do change your practice which I think is sensible. Clinicians are often criticised for not treating each patient as an individual and considering the material risks to them and how to mitigate them. If we have a scenario where the first eye underwent surgery and developed CMO which resolved in three months, and then the other eye was operated upon without any alteration of management, you could be criticised if the second eye subsequently failed to resolve. My question would be: “You knew the first eye developed it and you knew there were simple ways to try and stop the second eye developing it, so why didn’t you use them?” I give NSAID drops five days pre-op and carry them on in the postoperative period.
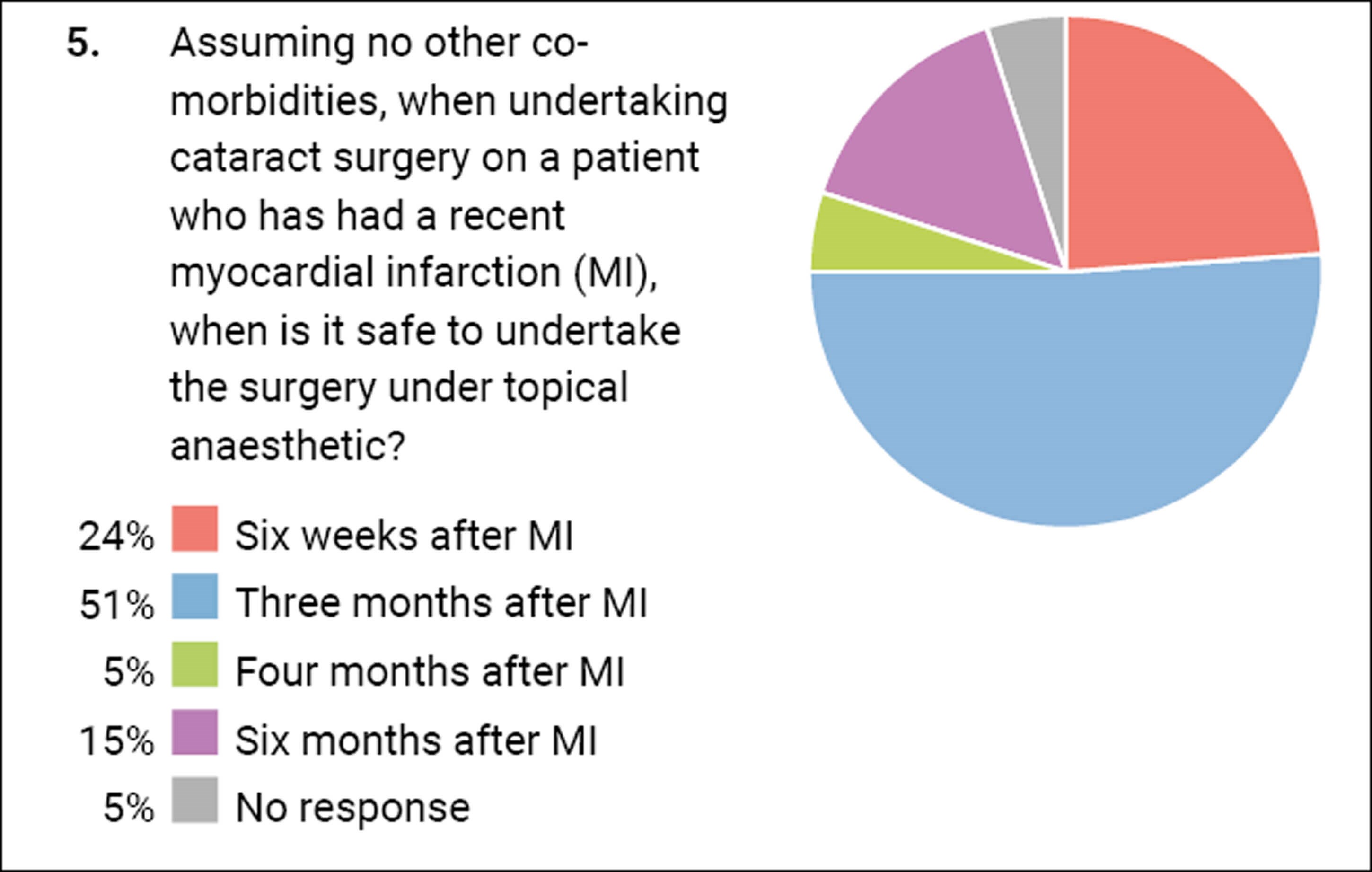
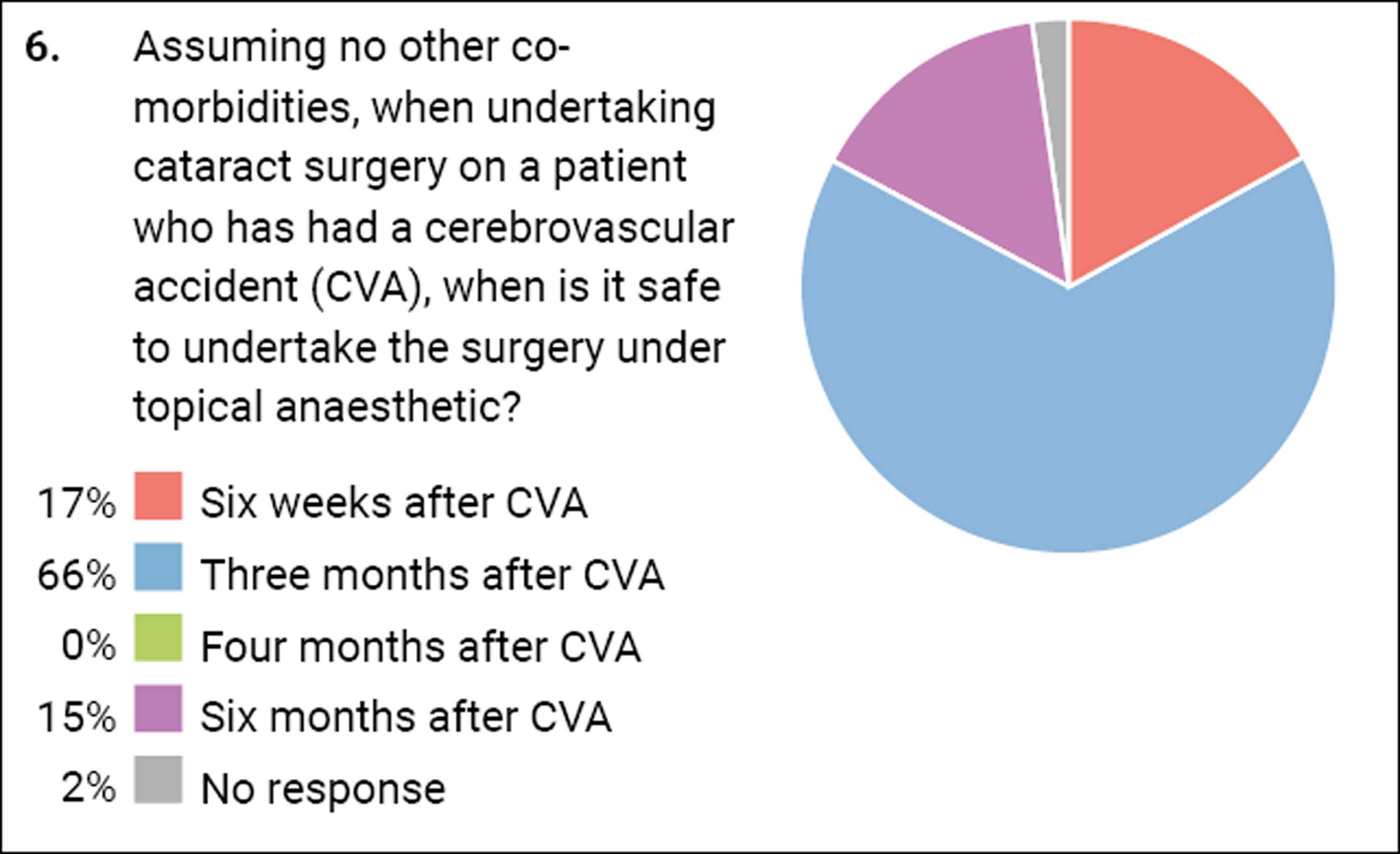
These next two questions highlight a postcode lottery which patients face. In one unit a patient may be allowed to have their cataract surgery six weeks after their myocardial infarction (MI) but the same patient would be refused it in another Trust until they were six months post MI. More than half of you went for three months as the cut-off period after which it is safe to have their cataract procedure. When looking at the same issue regarding a stroke, there was again variance, but three months seemed to be the magic number again.
Getting it Right First Time (GIRFT) has issued some guidance [8], which states:
“Elective cataract surgery should be postponed for three months following myocardial infarction, cardiac therapeutic intervention (e.g., stent or angioplasty), stroke or transient ischaemic attack (TIA). Patients who have experienced one of these conditions in the last three months are American Society of Anaesthesiologists (ASA) physical classification grade IV and are at high-risk of a subsequent event in the immediate post event period. Patients with angina should be advised to bring their usual angina medication and have this available to use throughout their admission including in theatre. Any patient with new or un-investigated chest pain should be sent for immediate review prior to elective cataract surgery.”
Therefore, it seems that three months is a consensus and certainly a timescale I will seek to adopt in my practice.
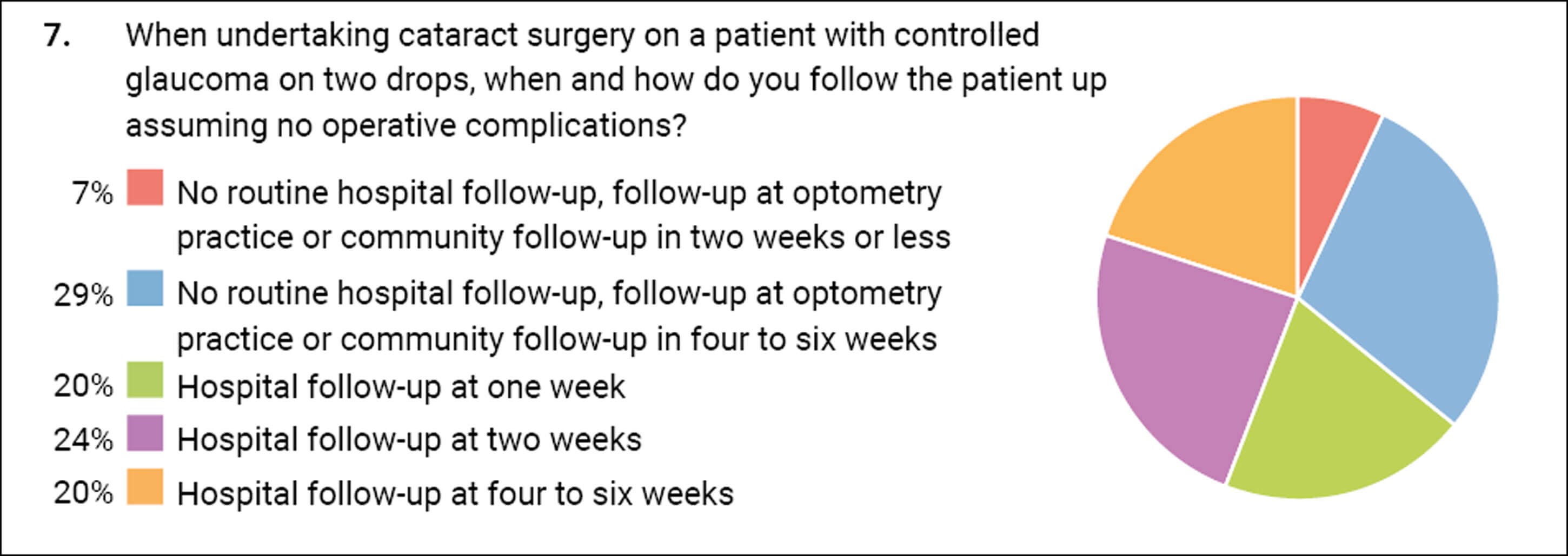
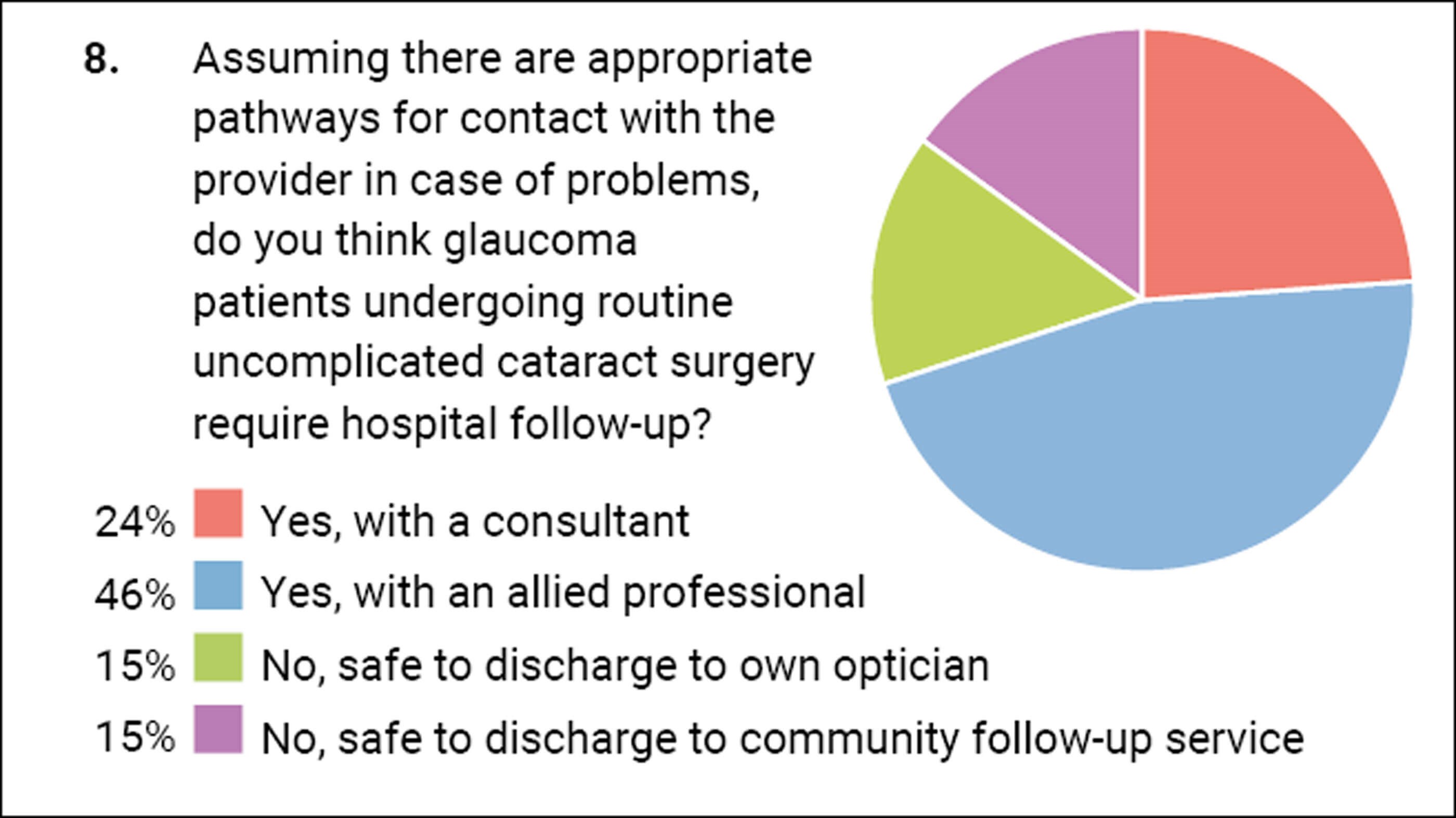
The follow-up of cataract patients or the lack thereof has been a matter of debate for some time. Historically, patients were routinely brought back on day one however the evidence for this was weak and gradually, as the safety profile of cataract surgery has improved, this is no longer standard practice. Many patients are now discharged to community follow-up services or back to their own optician. Since the Covid-19 pandemic there has been a drive to minimise hospital attendances unless required. Arguably glaucoma patients are a special group who may warrant closer attention in the postoperative period. The survey results show there is still some variance in what clinicians do with such patients. Two thirds felt that some sort of hospital follow-up was required but the timescale varied from 1–6 weeks. In the following question, one third felt that it was safe to discharge from hospital care with only community follow-up.
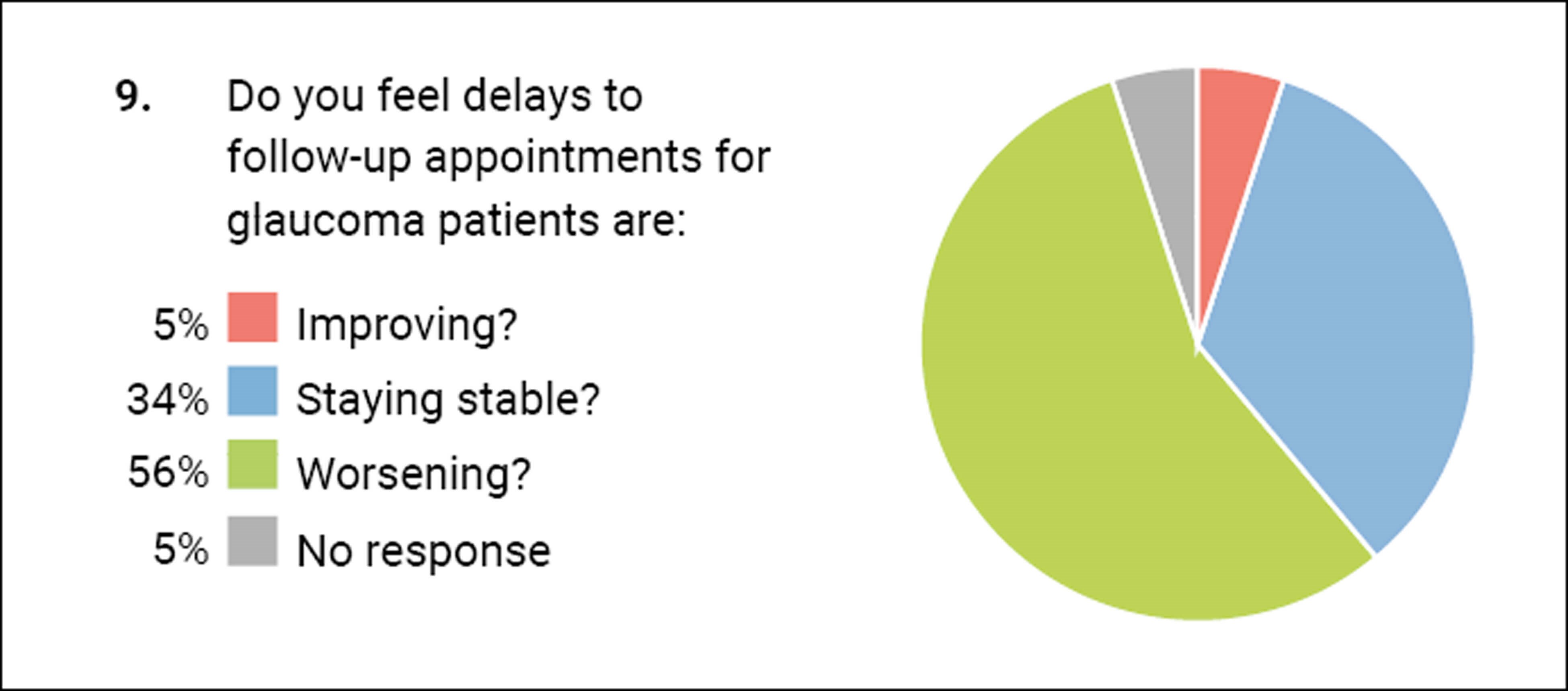
The last question sought to get a feel of where we are in the recovery post-Covid-19. It was upsetting and concerning to see that more than half of you felt that the delays to follow-up for glaucoma patients was worsening.
References
1. Kim SJ, Schoenberger SD, Thorne JE, et al. Topical nonsteroidal anti-inflammatory drugs and cataract surgery: a report by the American Academy of Ophthalmology. Ophthalmology 2015;122:2159–68.
2. Bellocq D, Pierre-Kahn V, Matonti F, et al. Effectiveness and safety of dexamethasone implants for postsurgical macular oedema including Irvine-Gass syndrome: the EPISODIC-2 study. Br J Ophthalmol 2017;101:333–41.
3. McCafferty S, Harris A, Kew C, et al. Pseudophakic cystoid macular edema prevention and risk factors; prospective study with adjunctive once daily topical nepafenac 0.3% versus placebo. BMC Ophthalmol 2017;17:16.
4. Wendel C, Zakrzewski H, Carleton B, et al. Association of postoperative topical prostaglandin analog or beta- blocker use and incidence of pseudophakic cystoid macular edema. J Glaucoma 2018;27:402–6.
5. Wielders LHP, Schouten J, Winkens B, et al. European multicenter trial of the prevention of cystoid macular edema after cataract surgery in nondiabetics: ESCRS PREMED study report 1. J Cataract Refract Surg 2018;44:429–39.
6. Flach AJ. The incidence, pathogenesis and treatment of cystoid macular edema following cataract surgery. Trans Am Ophthalmol Soc 1998;96:557–634.
7. Henderson BA, Kim JY, Ament CS, et al. Clinical pseudophakic cystoid macular edema. Risk factors for development and duration after treatment. J Cataract Refract Surg 2007;33:1550–8.
8. High flow cataract guides (applicable to surgery of any complexity, provided under local anaesthetic) Guide 4: Perioperative management of patients with medical comorbidities and additional needs (2023). Getting It Right First Time.
https://gettingitrightfirsttime.co.uk/
wp-content/uploads/2023/12/Guide-4
-Perioperative-management-of-patients
-with-medical-comorbidities-FINAL
-Updated-November-2023.pdf
[Link last accessed January 2024].
COMMENTS ARE WELCOME








