Annie SeeWah Tung provides an overview of toxoplasmosis retinochoroiditis, including guidance on which cases should be treated and the treatment options.
Toxoplasmosis retinochoroiditis is an infectious condition that is characterised by retinochoroidal lesions commonly in the posterior pole and is associated with posterior uveitis in its active form. A classic description of “headlight in fog” is given due to its identifiable bright yellowish ovoid lesions amongst the intense surrounding vitritis on fundus examination (Figure 1).
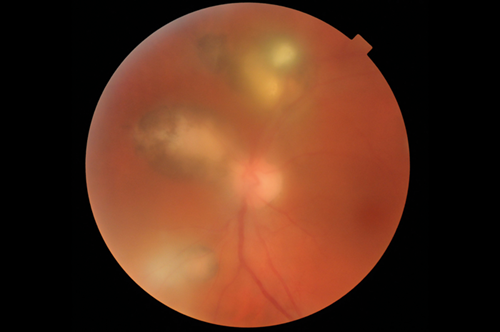
Figure 1: Toxoplasmosis retinochoroiditis: this 37-year-old male had two previous episodes.
Note the typical lesions, pigmented borders and the bright foci are newly active lesions. Visual acuity was 6/18.
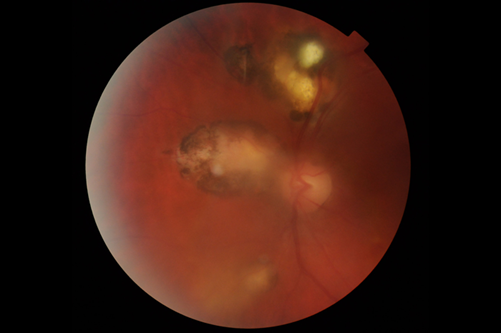
Figure 2: Same patient as in Figure 1 above. This is after treatment with co-trimoxazole 160/800mg andprednisolone 60mg.
A week later the following was added: Clindamycin 150mg QDS, tapering of oral prednisolone and gutt.
Dexamethasone 1% 6x daily. Visual acuity was 6/9.
Other common associated clinical features include stellate keratic precipitates, non-granulomatous iridocyclitis and raised intraocular pressure. Atypical presentations include scleritis, serous retinal detachments, retinal vasculitis, retinal vascular occlusions, neuroretinitis and optic neuritis. Like other inflammatory uveitis choroidal neovascularisation is possible. Ocular complications may manifest in children in the form of pigmentary retinopathy, choroidal neovascularisation, cataracts, glaucoma, optic nerve atrophy and retinal detachment [1]. Cerebral calcification, hydrocephalus and hepatosplenomegaly is also described in the congenital form.
Toxoplasmosis infection is caused by an obligate intracellular protozoa Toxoplasmosis gondii. To understand how to eliminate the parasite, we need to understand the lifecycle of the organism. T. gondii exists in three main forms: the oocysts, produced during the sexual cycle in the intestine of an infected feline and passed in cat faeces; the bradyzoites, tissue cysts that mainly persist in the neural and muscular tissue, and can persist throughout the life of the host; and the tachyzoites, the rapid multiplying form which can enter host cells by active penetration or phagocytosis [2]. The organism can either be acquired by ingesting food or water contaminated by cat excrement, eating undercooked meat harboring tissue cysts or by receiving a contaminated blood transfusion or organ transplantation. Congenital transmission of T. gondii is also possible by transplacental infection from mothers to fetus [3].
When to treat
Toxoplasmosis retinochoroiditis is generally a diagnosis made on clinical examination alone. Serological tests support the diagnosis and can give an indication whether the infection is acute (IgM positive) or whether previous infection has occurred (IgG positive). Not all cases of toxoplasmosis retinochoroiditis require treatment. A recent survey performed to investigate the practices and treatment of ocular toxoplasmosis amongst Brazilian Uveitis Specialists showed 67.9% of the physicians treated all active cases [4]. Absolute indications for treatment suggested in the survey included reduced visual acuity to less than 20/200 (equivalent to 6/60), severe vitreous inflammation and ocular disease during acquired infection. Other indications include macular-threatening lesions, active lesion near the vascular arcade and / or optic nerve, and immune-compromised patients.
Reactivation of disease can present in pregnancy due to its immune- compromised state; however, acquisition of primary infection during gestation poses a higher risk of vertical transmission [5]. Treatment should not only be considered to preserve the mother’s vision, but to prevent vertical transmission of the parasite to the foetus as well. It is reported that the risk of vertical transmission increases with gestational age, peaking at around 36 weeks. The proportion of off-springs that develop clinical signs, however, is inversely related to the gestational age of maternal seroconversion. Serological tests and amniotic fluid polymerase chain reaction (PCR) are used to aid diagnosis of Toxoplasmosis in pregnancy.
Treatment
A recent evidence-based review published in 2014 attempted to evaluate various treatment modalities of toxoplasmosis retinochoroiditis through a comprehensive literature search [6]. A huge clinical heterogeneity was found amongst these studies, varying in combination of antibiotic, treatment dosage, duration and frequency.
Systemic treatment
Classic triple therapy – this includes pyrimethamine (100mg OD loading then 25-50mg daily for 30-60 days), sulfadiazine (1g QDS) and folinic acid (5- 20mg OD).
Classic triple therapy works by inhibiting folic acid metabolism of the parasite and is therefore contraindicated in the first trimester of pregnancy. It is thought to be the most efficacious treatment but is limited by its adverse effects. Pyrimethamine causes dose related bone marrow suppression and requires weekly haematological tests while the patient is on treatment. A reduced dose is also recommended in patients with AIDS due to pre-existing bone marrow suppression and its antagonistic effect with zidovudine. Both pyimethamine and sulfadiazine can cause nausea and anorexia, but sulfadiazine is also associated with hepatotoxicity and severe reactions such as Steven-Johnson’s Syndrome.
“A study has suggested that T.gondii may affect human behavior by making infected women more intelligent, warm and outgoing while causing infected men to be more reflective, rigid and emotionally less stable! Read Kevin D Lafferty’s paper ‘Can the common brain parasite, Toxoplasma gondii, influence human culture’ to find out more about his research [18].”
Clindamycin (150mg-300mg QDS) – this can be used as a monotherapy, as part of the triple therapy by replacing pyrimethamine or in conjunction with the triple therapy to form the quadruple therapy.
It works by reducing toxoplasma cysts. A study by Rothova et al. in 1989 compared three triple-drug combinations: i) pyrimethamine, sulfadiazine and corticosteroids; ii) clindamycin, sulfadiazine and corticosteroids; iii) trimethoprim, sulfamethoxazole and corticosteroid. The results showed no difference in the duration of inflammation activity between the groups [7].
Trimethoprim / sulfamethoxazole (160/800mg OD) – also known as co-trimoxazole or Septrin, is a popular alternative treatment choice to the classic therapy.
A single-blinded prospective randomised clinical trial compared co-trimoxazole (with prednisolone) versus the classic treatment (with prednisolone). It showed no significant difference between reduction in lesion size and improvement in visual acuity between the two regimes. The overall recurrence rate after 24 months and adverse effect rate were similar [8]. Be wary of the side effects of co-trimoxazole such as hepatic derangement, agranulocytosis, aplastic anaemia, and other blood dyscrasias, similarily avoid in G6PD deficiency and known sulfonamide allergy.
Azithromycin (500mg OD) – A pilot study compared azithromycin monotherapy to classic treatment in non-vision threatening disease showed better treatment tolerance in azithromycin but clinically longer duration of treatment to disease inactivity [9]. Another study where azithromycin was used instead of sulfadiazine in triple therapy in a prospective randomised multicentre study showed similar efficacy in resolution time, decrease in lesion size and recurrence in the azithromycin arm compared to the classic triple therapy in sight-threatening disease [10].
While antibiotic therapy stops the multiplication of parasite, corticosteroids are commonly used as an adjunctive therapy. It is believed they reduce associated inflammation and minimise ocular tissue damage. Published evidence suggests a wide variation in initiation and duration of the use of corticosteroids. Some physicians use adjunctive steroids in all immuno-competent patients regardless of clinical findings and others reserve it for specific indications [11]. Time to initiation of treatment varies from within three days to a week after starting anti-parasitic therapy. The Cochrane systematic review in 2013 investigated the current evidence on the use of corticosteroids but did not identify evidence from randomised control trials for the role of its use [12]. The review recommended a conservative approach by initiating a starting dose of 1mg/kg/day. Dosage may be amended depending on side-effects and should be tapered rapidly when inflammation is adequately controlled by anti-parasitictherapy [12].
Intravitreal treatment
Intravitreal clindamycin 1mg/0.1cc – this can be used in macular threatening cases in pregnancy where classic therapy is contraindicated, or in patients who are intolerant to systemic treatment. The half-life of the drug lasts for 40 hours and can be co-injected with dexamethasone 400μg. Injections can be repeated fortnightly. A masked prospective trial in 2011 compared intravitreal clindamycin and dexamethasone (IVCD) to the classic therapy with oral steroids. The trial showed no significant difference in visual acuity improvement, but IgM positive cases responded better to classic treatment in terms of lesion size reduction. Fewer systemic side-effects were reported in the intravitreal group [13].
“The decision to treat active infection in pregnancy poses additional dilemma due to treatment toxicity and potential teratogenicity.“
Treatment in pregnancy
The decision to treat active infection in pregnancy poses additional dilemma due to treatment toxicity and potential teratogenicity. Pyrimethamine should be avoided in the first trimester as it works by inhibiting folic acid metabolism. Some also suggest avoiding the use of sulphonamides in the third trimester due to risk of causing kernicterus [14]. Antimicrobials including clindamycin, azithromycin have shown to be a safe alternative. Disease and treatment monitoring should be performed in conjunction with the care of the obstetrician. Spiramycin 1g TDS – Spiramycin is not known to be teratogenic. Studies have reported a reduction of vertical transmission with the use of spiramycin; however, as it does not cross the placenta, it is not a reliable treatment for infected foetus [5].
Long-term prophylaxis
Recurrence of toxoplasmosis retinochoroiditis is not uncommon and was observed in up to 45.2% of cases in a prospective observational study [15]. Rupture of dormant retinal cysts is thought to be related to recurrences, however, the exact cause remains unclear [1]. Intermittent use of trimethoprim 160mg / sulfamethoxazole 800mg every three days was found to have a lower recurrence rate in patients suffering from recurrent disease compared to controls [16]. This is recommended by some specialist as secondary prophylaxis in selected immunocompetent patients [17].
References
1. Commodaro AG, Belfort RN, Rizzo LV, et al. Ocular toxoplasmosis: an update and review of the literature. Memórias do Instituto Oswaldo Cruz 2009;104(2):345-50.
2. Dubey JP, Lindsay DS, Speer CA. Structures of toxoplasma gondiitachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clinical Microbiology Reviews 1998;11(2):267-99.
3. Parasties – Toxoplasmosis (Toxoplasma infection). Centres of disease control and prevention:
http://www.cdc.gov/parasites/toxoplasmosis/biology.html
Last accessed February 2017.
4. Morais FB, Arantes TE, Muccioli C. Current practices in ocular toxoplamosis: a survey of Brazilian uveitis specialists. Ocul Immunol Inflamm 2016 Sep:1-7 [Epub ahead of print].
5. Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis 2008;47(4):554-66.
6. Harrell M, Carvounis PE. Current treatment of toxoplasma retinochoroiditis: an evidence-based review. J Ophthalmol 2014;2014:273506.
7. Rothova A, Buitenhuis HJ, Meenken C, et al. Therapy of ocular toxoplasmosis. Int Ophthalmol 1989;13:415-9.
8. Soheilian M, Sadoughi MM, Ghajarnia M, et al. Prospective randomized trial of trimethoprim / sulfamethoxazole versus pyrimethamine and sulfadiazine in the treatment of ocular toxoplasmosis. Ophthalmology 2005;112:1876-82.
9. K Balaskas, Vaudaux J, Boillat-Blanco N, Guex-Crosier Y. Azithromycin versus Salfadiazine and Pyrimethamine for non-vision threatening toxoplasmicretinochoroiditis: a pilot study. Med Sci Monit 2012;18(5):CR296-302.
10. Bosch-Driessen LH, Verbraak FD, Suttorp-Schulten MS, et al. A prospective, randomised trial of pyrimethamine and azithromycin vs. pyrimethamine and sulfadiazine for the treatment of ocular toxoplasmosis. AJO 2002;134(1):34-40.
11. Holland GN, Lewis KG. An update on current practices in the management of ocular toxoplasmosis. Am J Ophthalmol 2002;134(1):102-14.
12. Jasper S, Vedula SS, John SS, et al. Corticosteroids for ocular toxoplasmosis. Cochrane Database Syst Rev 2013;4:CD007417.
13. Soheilian M, Ramezani A, Azimzadeh A, et al. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology 2011;118:134-41.
14. Nath R, Guy E, Morrison A, Kelly SP. Toxoplasma retinochoroiditis in pregnancy: Using current evidence to inform management. Clinical Ophthalmology (Auckland, NZ) 2009;3:657-61.
15. Aleixo AL, Curi ALL, Benchimol EI, Amendoeira MRR. Toxoplasmic retinochoroiditis: clinical characteristics and visual outcome in a prospective study. PLoS Negl Trop Dis 2016;10(5):e0004685.
16. Silveira C, Belfort R Jr, Muccioli C, et al. The effect of long-term intermittent trimethoprim/sulfamethoxazole treatment on recurrences of toxoplasmicretinochoroiditis. Am J Ophthalmol 2002;134:41-6.
17. Holland GN. How do I treat a patient with ocular toxoplasmosis. Curbside consultation in Uveitis: 49 Clinical Questions:
http://www.healio.com/ophthalmology/curbside-consultation/
%7B3193bbb0-fed5-46c9-841b-81bd310a198c%7D/
how-do-i-treat-a-patient
Last accessed February 2017.
18. Lafferty KD. Can the common brain parasite, Toxoplasma gondii, influence human culture?
Proc Biol Sci 2006;273(1602):2749-55.
TAKE HOME MESSAGE
-
Not all cases of ocular toxoplasmosis retinochoroiditis require treatment.
-
Classic triple therapy has the highest side- effect profile.
-
Alternative treatment includes clindamycin, azithromycin, co-trimoxazole which have similar efficacy and are better tolerated.
-
Corticosteroid should not be used in immunocompromised patients and should commence after the initiation of antibiotic treatment.
-
Triple therapy should be avoided in the first trimester of pregnancy.
COMMENTS ARE WELCOME





