Uveal melanoma is the most common primary intraocular tumour. However, they are still rare, with an incidence of 2-8 per million [1].
The presence of a choroidal naevus is a risk factor for uveal melanoma [1]. Patients with choroidal lesions are typically asymptomatic, hence suspicious lesions are usually detected on routine examination and followed up with further imaging. Ocular tumours in the UK are managed at one of four specialist tertiary ocular oncology centres located in London, Liverpool, Sheffield, and Glasgow [2,3].
Uveal melanoma most commonly presents in the choroid (85-90%) and less commonly in the ciliary body (5-8%) or iris (3-5%) [4-6]. Incidence of uveal melanoma varies by sex, race, and country – males and Caucasians have a higher incidence. In Europe, incidence increases with latitude. This ranges from two per million in Italy, four-six per million in central Europe and greater than eight in Denmark and Norway. The peak age of incidence is 60 for fair-skinned patients. At least 10% of uveal melanomas develop from a naevus, making it the most common risk factor [6]. The prevalence of choroidal naevus in the population is 5%, of which 1% transform into melanoma [6,7].
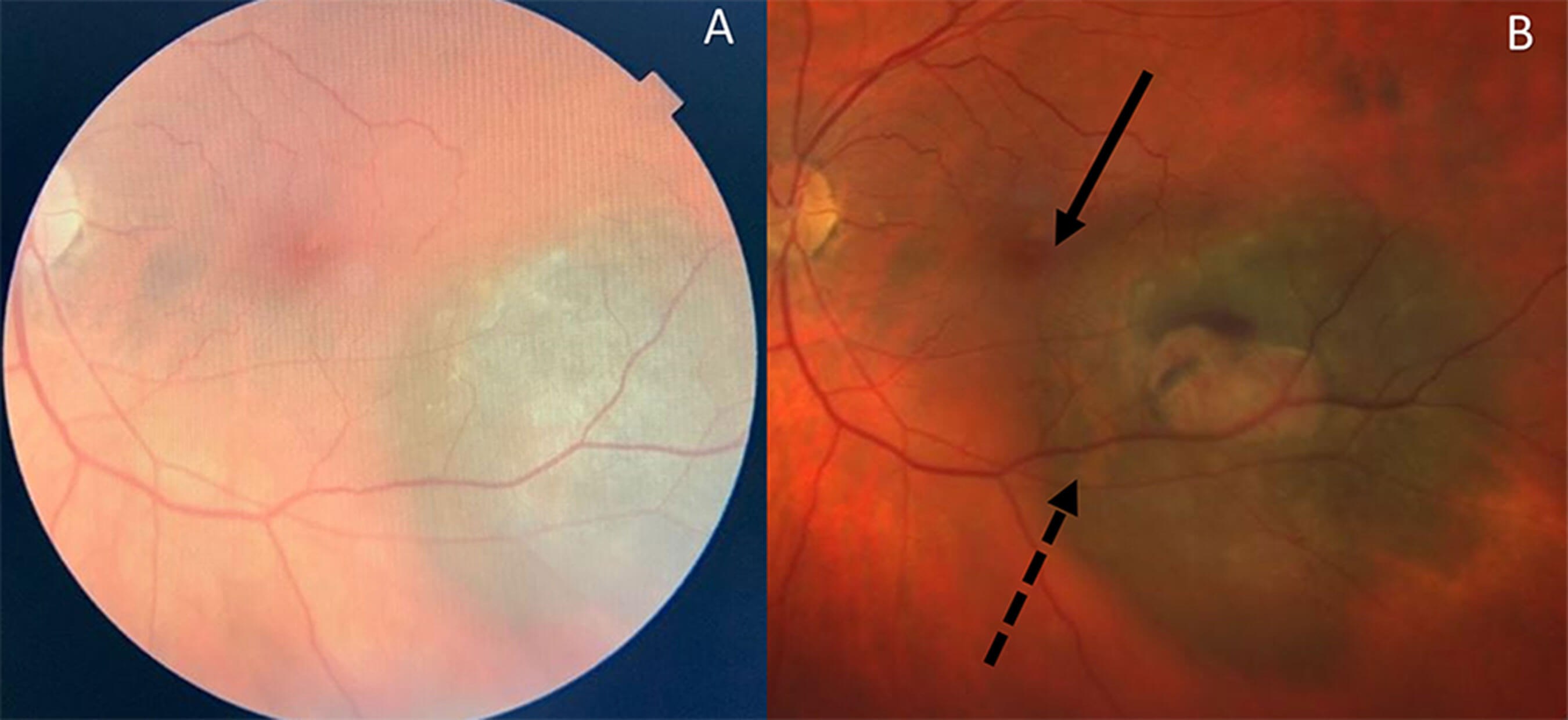
Figure 1: A: Optician fundus photograph with left indeterminate choroidal lesion; B: Following 10 years of observation, transformation into a left choroidal melanoma (AJCC Stage 1). Lesion enlarged towards fovea [solid arrow] causing visual symptoms. Orange pigment represents lipofuscin [dash arrow] which is a risk factor for transformation. [Note: fundus photographs are from different retinal imaging devices]
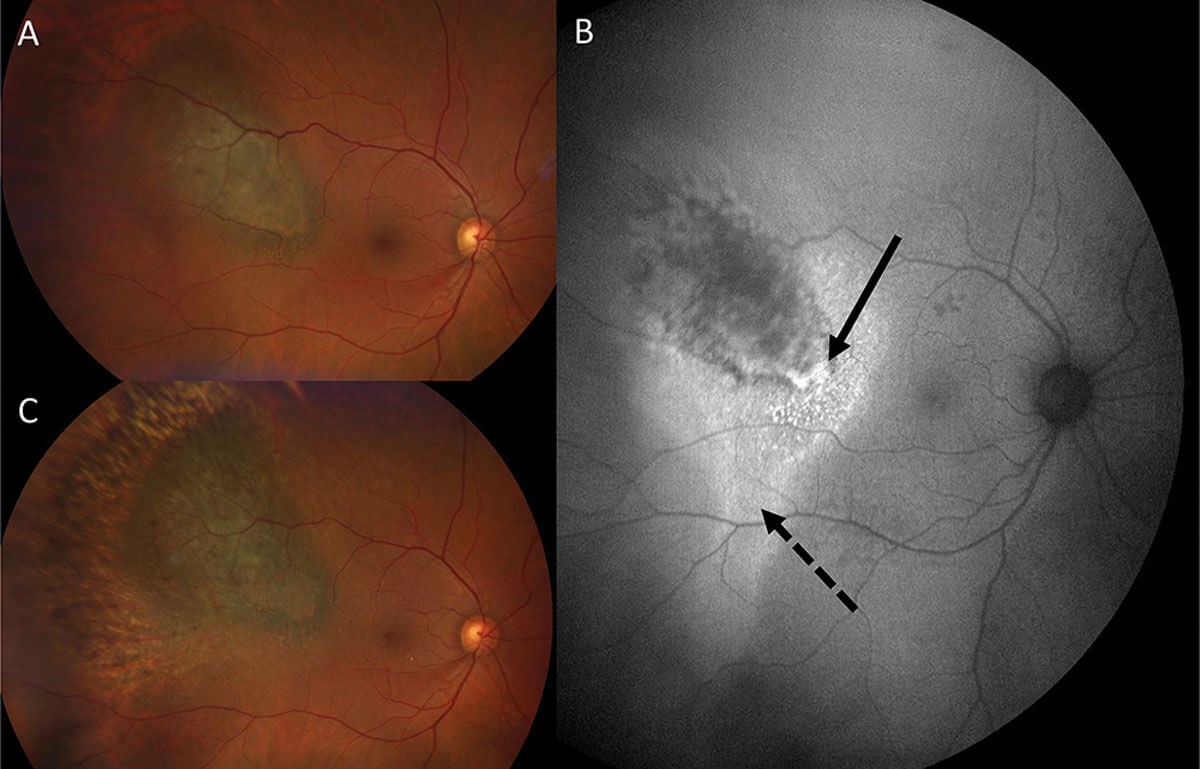
Figure 2: A: Right choroidal melanoma (AJCC Stage 1); B: Fundus Autofluorescence highlights lipofuscin (solid arrow) and subretinal fluid (dash arrow).C: Six months after Ruthenium Plaque Brachytherapy, choroidal melanoma shows surrounding post-radiation atrophy. Following treatment, thechoroidal lesion thickness decreased on BScan Ultrasound and subretinal fluid resolved on Ocular Coherence Tomography (OCT).
Clinical features
Choroidal melanomas can be visualised on fundal examination. They can be pigmented, amelanotic or have variable pigmentation. Patients may be asymptomatic, however symptoms such as photopsia, scotoma or blurred vision can be experienced. The prevalence of these at presentation is dependent on the lesion size and location. There are certain characteristics which distinguish between a naevus and melanoma. On fundoscopy, naevi often appear as oval or circular, brown / grey, relatively flat lesions. They typically have overlying drusens and depigmented halo. Features such as the presence of orange pigment, lipofuscin, and subretinal fluid increase suspicion for melanoma [8].
The mnemonic “To Find Small Ocular Melanoma Doing IMaging” (TFSOM-DIM) is useful to assess the risk of neavus growth into melanoma [9].
- Thickness: >2mm (ultrasound)
- Fluid: subretinal (OCT)
- Symptoms: vision loss / photopsia (visual acuity)
- Orange pigment: (autofluorescence)
- Melanoma: low internal reflectivity / acoustically hollow (ultrasonography) and
- DIameter: size >5mm (fundus photography).
The most significant risks of choroidal neavus growth into melanoma occur with an increased combination of risk factors: mean 5-year estimates of neavus growth into melanoma are 1% for those with zero risk factor, 11% with one factor, 22% with two factors, 34% with three factors, >50% with four or more factors [9].
Most ocular oncologists now place less importance on proximity to the optic nerve as a significant risk factor. The basal dimensions of the lesion are now a recognised risk factor for transformation, and are also a principal consideration to guide clinical staging and treatment modality [9,10]. Negative risk factors such as drusen or surrounding halo are clinically relevant, with these findings more consistent with choroidal naevi or chronic, slow-growing lesions [11].
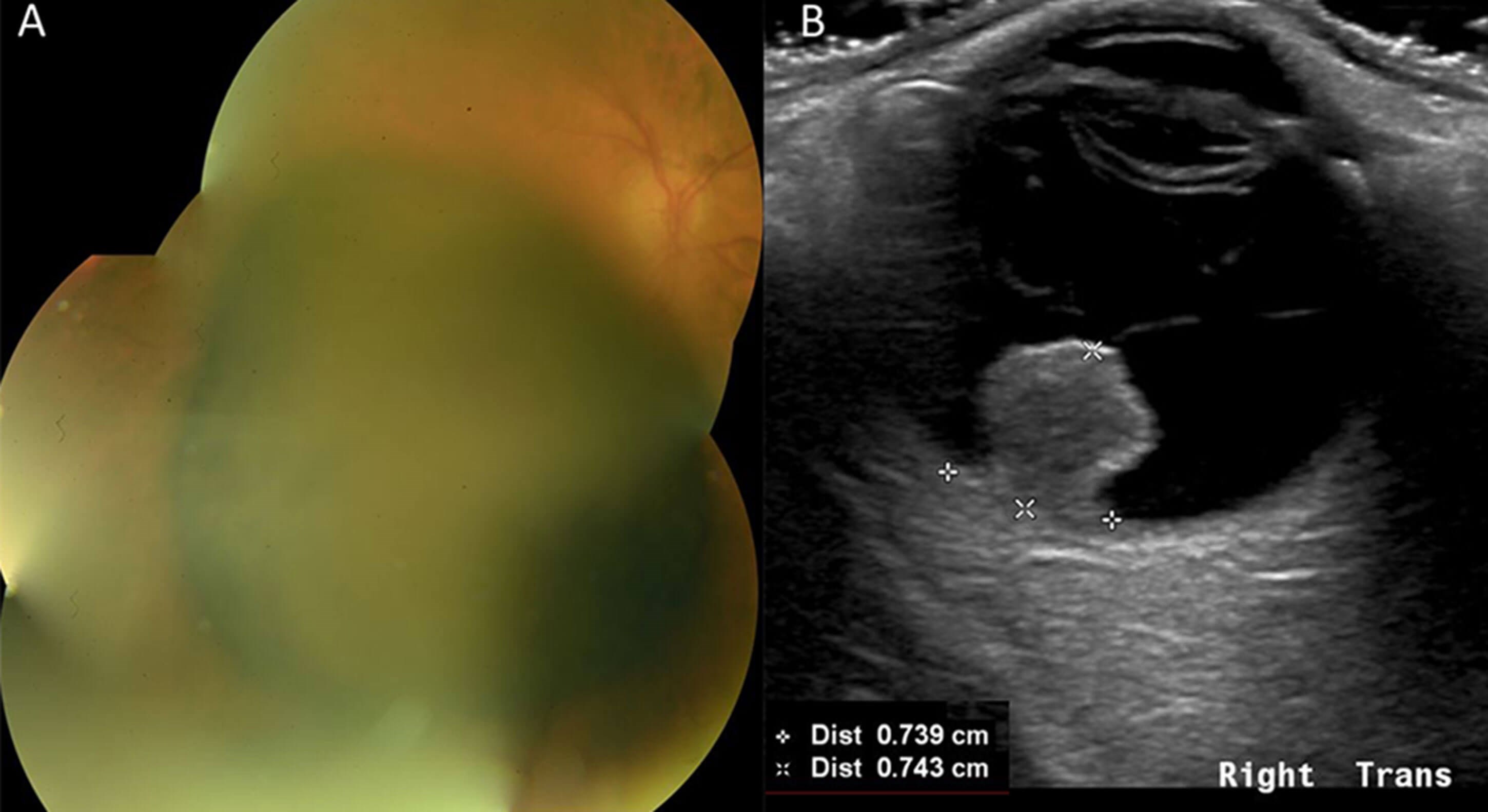
Figure 3: Right choroidal melanoma (AJCC Stage 2A); A: Large pigmented choroidal mass; B: Eye Ultrasound Scan highlights collarstud morphology,with Proton Beam Therapy (PBT) Measurements. [Basal dimensions 8.0 x 7.4mm. Maximum thickness 7.4mm]. This choroidal melanoma responded well to PBT with reduction in basal dimensions and thickness.
The management and surveillance of choroidal naevi can be undertaken by optometrists and ophthalmologists. There is no formalised surveillance program, yet the MOLES risk stratification system appears to be a sensitive tool for indicating malignancy [12,13]. Mushroom shape, Orange Pigment, Large Size, Enlargement and Subretinal fluid (MOLES) are the risk factors used for the scoring. This scoring system can be used by optometrists to decide if the patient needs referral to an ophthalmologist for review and as a guidance to determining frequency of follow up.
A score of zero indicates common naevus, which can be monitored by optometrist in the community. Score of one is 'low-risk naevus' which can be monitored by an optometrist or ophthalmologist. Score of two is 'high-risk naevus' which requires monitoring by an ophthalmologist. Score of three or more is 'probable melanoma' and requires urgent referral to an ocular oncologist. The inclusion of fundus photography with referral is of particular importance to guide clinical triage and expedite specialist assessment [14].
Investigations
Imaging and special tests are required to confirm the diagnosis. Ultrasound is the preferred investigation for evaluating and monitoring uveal tumours. Ultrasound biomicroscopy (UBM) can be used to detect tumours of the iris and ciliary body. Optical coherence tomography (OCT) is commonly performed to evaluate the posterior segment and is valuable in measuring small tumours and subretinal fluid [2]. Autofluorescence can confirm clinical assessment of lipofuscin or subretinal fluid. Angiography (fluorescein, indocyanine green) can evaluate tumour vasculature and assess for radiation retinopathy following brachytherapy. If these less invasive methods cannot differentiate the type of lesion, then biopsies can be performed, although they tend to be used more for prognostic information rather than diagnostic purposes [3,14,15]. Whole-genome sequencing (WGS) of biopsy samples has been utilised to improve knowledge of uveal melanoma genomics and to identify potential therapeutic targets [16].
Staging
The American Joint Committee on Cancer (AJCC) classification system is used to stage uveal melanoma. The staging is calculated using the TNM system and used as the prognostic parameter. Tumour location, thickness, largest basal diameter, ciliary body involvement and extra-scleral extension form the basis of the tumour category score [10].
Treatment
The uveal melanoma UK national guidelines state the primary outcomes from management are survival / distal recurrence, preserving the eye and preserving vision. Secondary outcomes are quality of life, visual acuity, local recurrence and side-effects [3]. Traditional treatment included primary enucleation, however, with advancements in radiotherapy, eye preserving techniques are standard practice where appropriate. Management of primary uveal melanoma can be divided into globe preserving or enucleation [6]. These can be further subclassified into surgery, radiotherapy, and laser therapy.
If appropriate, brachytherapy is often the first choice of treatment and involves attaching a radioactive plaque (ruthenium or iodine) to the episclera, which delivers a fixed dose of radiation. Small and medium-sized melanoma have five-year control rates of >95% [3]. Side-effects include visual complications if the radiation damages adjacent structures. For larger tumours, proton beam therapy (PBT) and stereotactic radiosurgery (SRS) are treatment options. Both techniques use computerised treatment planning software to locate and irradiate tumours. PBT achieves more targeted radiation to the tumour. Under general anaesthetic, metal markers made from tantalum are sutured onto the eye and measurements of the tumour are recorded. The markers, ultrasonography and fundus photographs, are used to create a 3D model of the eye and calculate a precise radiation plan. SRS uses gamma rays with magnetic resonance imaging (MRI) or computerised tomography (CT) to map the area where radiation is required. This can all be done under local anaesthetic in a single session [3,17].
Transpupillary thermotherapy (TTT) is a laser treatment option for uveal melanoma. A beam of 1.2mm to 3mm is directed at the tumour and the heat from the laser induces tumour necrosis. TTT is successful for small uveal melanomas as a primary treatment. It is however used more frequently as an ancillary treatment to brachytherapy, known as sandwich therapy. The combination treatment can provide better local control, but studies have shown long-term visual outcome was worse than brachytherapy alone [3,18].
Another form of laser therapy which is not widely used is photodynamic therapy (PDT). The technique involves the intravenous administration of a photosensitising substance called verteporfin. Afterwards, a laser is targeted at the lesion, which activates verteporfin leading to free radial formation and destruction of the tumour. Studies show that PDT could be an effective primary treatment for small amelanotic choroidal melanomas [19].
The most common surgery performed for local tumour control is enucleation. It is performed if other treatments are not successful, however, can also be offered as primary treatment. It provides 100% local tumour control if completely excised. With the use of an orbital implant and artificial eye, cosmetic results are good. Procedures such as exoresection and endoresection attempt to remove the tumour surgically whilst preserving the eye. Adjunctive radiotherapy or brachytherapy is typically implemented for better local control. These surgical techniques however are challenging and not commonly used in the UK [3].
Metastases
The liver is the most common site for developing metastasis, accounting for 77-94% of lesions [20]. Median survival after developing metastasis is 6-12 months. Overall, patients have a relative survival of 60% 15-20 years after diagnosis of uveal melanoma [21]. There is no current, established standard of care for metastatic disease, however current attempts involve systemic treatment (chemotherapy, immunotherapy, and targeted therapy) and liver-directed therapy (resection, radioembolisation, chemo-embolisation, and percutaneous hepatic perfusion) [6,22].
It has been suggested that detecting uveal melanomas early can improve patient outcomes. However, there is no consensus across the world about the duration, frequency, or mode of hepatic surveillance. In Europe, liver ultrasounds are the most common modality used for metastatic surveillance. MRI is considered to be the more sensitive imaging modality for the liver whereas CT or positron emission tomography (PET) are sensitive for extrahepatic disease. In Scotland, a consensus statement has been developed to guide the metastatic surveillance of uveal melanomas by dividing them into low-to-medium risk and high-risk groups [20].
There is an unmet need for effective treatment for late-stage disease. Immune checkpoint inhibitors have revolutionised treatment for cutaneous melanoma, but as uveal melanoma is biologically different, it is not as efficacious on the latter. As most of the deaths from uveal melanoma are from liver failure, there needs to be continued research into liver directed therapy. Treatments developed could also have scope to be effective in other cancers that metastasise in the liver. Ongoing clinical trials, especially looking into T-cell therapy are showing promising results. Advancements in basic science research are developing our understanding of tumour pathways and vulnerabilities. As we further understand the mechanism of disease, novel treatments can be developed to improve patient outcomes [3,22,23].
Conclusion
Uveal melanoma is a rare malignancy which can be picked up on clinical examination and further classified with imaging modalities. Specialist ocular oncology referral and assessment is important to guide management. Treatment of the primary ocular tumour can be in the form of surgery, radiotherapy, or laser therapy. Surveillance is an important part of management as a high proportion of patients develop metastases, primarily in the liver. Therapeutic options for metastatic uveal melanoma are limited, however systemic- and / or liver-directed treatment options are available for appropriate patients. Further research in the understanding of genetics and tumour pathways is showing promising results for the development of novel treatments.
References
1. Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology 2005;112(10):1784–9.
2. Salmon JF. Kanski’s Clinical Ophthalmology. 9th Edn. Amsterdam, NL; Elsevier; 2021.
3. Nathan P, Coupland S, Cohen V, et al. Uveal Melanoma UK National Guidelines. Eur J Cancer 2015;51(16):2404-12.
4. McLaughlin CC, Wu XC, Jemal A, et al. Incidence of noncutaneous melanomas in the U.S. Cancer 2005;103(5):1000-7.
5. Damato EM, Damato BE. Detection and time to treatment of uveal melanoma in the United Kingdom: an evaluation of 2,384 patients. Ophthalmology 2012;119(8):1582-9.
6. Krantz BA, Dave N, Komatsubara KM, et al. Uveal melanoma: epidemiology, etiology, and treatment of primary disease. Clin Ophthalmol 2017;11:279.
7. Williams BK Jr, Di Nicola M. Ocular Oncology-Primary and Metastatic Malignancies. Med Clin North Am 2021;105(3):531-50.
8. Shields CL, Shields JA, Kiratli H, et al. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Trans Am Ophthalmol Soc 1995;93:259-79.
9. Shields CL, Dalvin LA, Ancona-Lezama D, et al. Choroidal Nevus Imaging Features in 3,806 Cases and Risk Factors for Transformation into Melanoma in 2,355 Cases: The 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina 2019;39(10):1840-51.
10. Updated AJCC Classification for Posterior Uveal Melanoma. Retina Today.
https://retinatoday.com/articles/2018
-may-june/updated-ajcc-classification
-for-posterior-uveal-melanoma
11. Shields CL, Furuta M, Berman EL, et al. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol 2009;127(8):981-7.
12. Pigmented fundus lesions. College of Optometrists.
https://www.college-optometrists.org/
clinical-guidance/clinical-management-guidelines/
pigmentedfunduslesions#Evidence
13. Roelofs KA, O’day R, Harby LA, et al. The MOLES System for Planning Management of Melanocytic Choroidal Tumors: Is It Safe? Cancers (Basel) 2020;12(5):1311.
14. Foti PV, Travali M, Farina R, et al. Diagnostic methods and therapeutic options of uveal melanoma with emphasis on MR imaging—Part I: MR imaging with pathologic correlation and technical considerations. Insights Imaging 2021;12(1):1-27.
15. Jacobsen BH, Ricks C, Harrie RP. Ocular ultrasound versus MRI in the detection of extrascleral extension in a patient with choroidal melanoma. BMC Ophthalmol 2018;18(1):1-4.
16. Johansson PA, Brooks K, Newell F, et al. Whole genome landscapes of uveal melanoma show an ultraviolet radiation signature in iris tumours. Nat Commun 2020;11(1):2408.
17. Sikuade MJ, Salvi S, Rundle PA, et al. Outcomes of treatment with stereotactic radiosurgery or proton beam therapy for choroidal melanoma. Eye 2015;29(9):1194-8.
18. Bornfeld N, Anastassiou G. Laser Treatment of Choroidal Melanoma. Retina 4th Edn. 2005;27:1-3:791-9.
19. Yordi S, Soto H, Bowen RC, Singh AD. Photodynamic therapy for choroidal melanoma: What is the response rate? Surv Ophthalmol 2021;66(4):552-9.
20. Chadha V, Cauchi P, Kincaid W, et al. Consensus statement for metastatic surveillance of uveal melanoma in Scotland. Eye (Lond) 2022;1-6.
21. Stålhammar G, Herrspiegel C. Long-term relative survival in uveal melanoma: a systematic review and meta-analysis. Commun Med 2022;2:18.
22. Carvajal RD, Schwartz GK, Tezel T, et al. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol 2017;101(1):38.
23. Chua V, Mattei J, Han A, et al. The Latest on Uveal Melanoma Research and Clinical Trials: Updates from the Cure Ocular Melanoma (CURE OM) Science Meeting (2019). Clin Cancer Res 2021;27(1):28-33.
[All links last accessed August-December 2022].
COMMENTS ARE WELCOME










