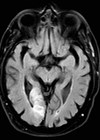
We present the case of a 12-year-old child presenting with a few days history of left-sided visual loss. Upon further investigation with magnetic resonance imaging (MRI) she was unexpectedly diagnosed with a right-sided chronic posterior cerebral arterial territory infarct, causing a left-sided homonymous hemianopia (HH). We hope to use this case to highlight some of the aetiologies of HH, and draw attention to the unusual presentation of stroke in children.
Case report
A 12-year-old girl presented to her general practitioner with new-onset visual loss in the left field of both eyes for one week. She had first noticed this visual field loss one day in class, however, felt the problem had been there for a while. She was immediately referred to the local ophthalmology department where she was diagnosed with left-sided HH. Examination of the anterior and posterior segments of the eye were unremarkable. She was referred onwards to the emergency department, however, as she was otherwise systemically well with no other symptoms she was discharged home to return two days later for an MRI scan of her head.
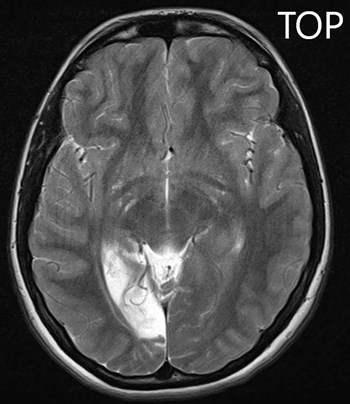
Figure 1: A T2-weighted MRI image of the patient’s brain,
demonstrating hyper-intensity in the right occipital lobe region.
The MRI scan (Figure 1) showed cortical and subcortical white matter volume loss in the right occipital lobe with secondary encephalomalacia, along with a focal area of cystic change within the right ventero-lateral thalamus. These findings led to a diagnosis of a chronic posterior cerebral artery territory infarct involving the right occipital lobe and posterior thalamus. She was commenced on Aspirin 75mg once-daily and admitted to a tertiary hospital for further review.
The patient had never had any visual problems before. She had been born full term via emergency Caesarean section due to meconium staining. At four-years-old she developed a right-sided viral pneumonia that led to secondary chronic lung changes, however, is managing well with normal exercise tolerance and very infrequent chest infections. There was no other past medical history.
Relevant family history included her maternal uncle having a stroke at 36-years-old, with no definitive aetiological cause found (other than slightly high / borderline cholesterol). Her father additionally suffers with migraines.
Aetiological investigations for the patient’s stroke are ongoing, with echocardiography showing a potential small patent foramen ovale, which could offer a possible explanation for her stroke. Otherwise, blood tests, electrocardiogram and computed tomography angiogram have shown no other cause for the infarct.
Comment
HH is a worrying symptom that should always prompt further investigation for an underlying cause [1]. Broadly, it can be caused by a variety of pathologies, resulting in brain lesions posterior to the optic chiasm in the visual pathway (Figure 2).
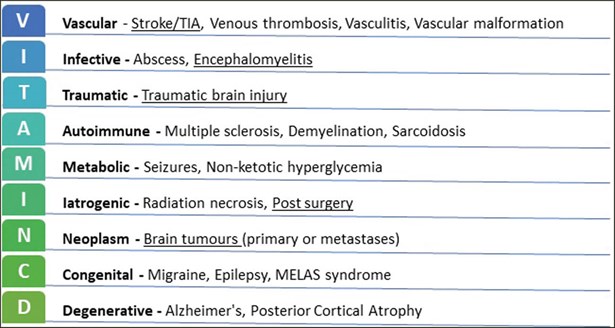
Figure 2: An example of a surgical sieve method of diagnosis using the commonly used VITAMIN CD acronym.
Included is a non-exhaustive list of some of the causes of HH, with the more common causes underlined [3,10].
Stroke (particularly infarction) is the leading cause of HH in adults, accounting for up to 70% cases of HH, with approximately 10% of all adult stroke patients suffering permanent HH [2]. However, a large study analysing 81 paediatric cases over 15 years found traumatic brain injury and tumours were the leading causes of HH in children, with infarction only accounting for 23% of cases [3].
In children, stroke can act as an early manifestation of underlying congenital disease, therefore a comprehensive family history is very important (as highlighted with this case).
Children with brain lesions will often not complain of visual problems or, such as in this instance, present in a delayed manner. Therefore, all children with brain lesions should undergo comprehensive visual testing (including field testing via confrontation examination or more formal Goldmann or automated perimetry).
HH can be very debilitating, with patients potentially experiencing considerable difficulty in everyday activities such reading, driving and general mobility [4]. Thankfully in the case of our patient it appears that she is tolerating any residual visual defect very well (being able to continue with dance classes and normal school activity, and with no reports of bumping into objects while mobilising or difficulties in reading).
Little is known about prognosis regarding resolution of HH in children, however, the aforementioned case series of 81 paediatric cases included follow-up formal field testing for 31 patients. In this subset of patients, improvement in HH was observed in 39% of children (which is consistent with figures of improvement in adults with HH) [3].
For those patients without significant improvement in their visual field and troublesome symptoms as a result, there are broadly three main methods to offset deficits [2,4,5]:
- Compensatory head and eye movements naturally developed by the patient (e.g. head-tilt, strabismus, altered saccadic movements etc.)
- Prism therapy to direct light away from the blind spots towards the retinal regions serving the visible hemi-fields
- Visual rehabilitation / training.
Visual rehabilitation is one area any clinician can assist the patient with, by being able to signpost them towards patient-centred visual training independent of formal rehabilitation organised by therapy teams. While there is no set recommended regime, there are various studies showing the benefit of visual training in HH patients (in real-world tasks such as visual search and reading) [5-7]. Two freely available examples are the Eye-Search and Read-right websites [8,9]. Both are linked to University College London (UCL) and based on research conducted on visual training exercises specifically for HH patients. These exercises have proven effective in improving visual function in initial studies, and patients’ involvement in such programmes can help to provide valuable data for further research.
References
1. Haaga M, Trauzettel-Klosinski S, Krumm A, et al. Homonymous Hemianopia in Children and Adolescents: An MRI Study. Neuropediatrics 2018;49:142–9.
2. Goodwin D. Homonymous hemianopia: challenges and solutions. Clin Ophthalmol Auckl NZ 2014;8:1919-27.
3. Kedar S, Zhang X, Lynn MJ, et al. Pediatric Homonymous Hemianopia. J Am Assoc Pediatr Ophthalmol Strabismus 2006;10:249-52.
4. Elfeky A, D’Août K, Lawson R, et al. Biomechanical adaptation to post-stroke visual field loss: a systematic review. Syst Rev 2021;10:84.
5. Ivanov IV, Kuester S, MacKeben M, et al. Effects of visual search training in children with hemianopia. PLoS ONE 2018;13:e0197285.
6. Woodhead ZVJ, Ong Y-H, Leff AP. Web-based therapy for hemianopic alexia is syndrome-specific. BMJ Innov 2015;1.
7. Ong Y-H, Jacquin-Courtois S, Gorgoraptis N, et al. Eye-Search: A web-based therapy that improves visual search in hemianopia. Ann Clin Transl Neurol 2015;2:74-8.
8. University College London Institute of Neurology. Eye-Search Therapy.
https://www.eyesearch.ucl.ac.uk
9. University College London Institute of Neurology. Read-Right – Hemianopic Alexia Therapy.
https://www.readright.ucl.ac.uk/
10. Wolberg A, Kapoor N. Homonymous Hemianopsia. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2021.
(All links last accessed December 2021)
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME