Introduction
The vast majority of ophthalmology units utilise allied healthcare professionals (AHPs) to deliver intravitreal injections (IVIs). The Royal College of Ophthalmologists issued a statement 10 years ago advocating the use of non-medical practitioners performing IVIs [1].
The main benefit being to expand capacity in a setting of ever-increasing demand for IVIs. Moorfields Eye Hospital forecasts up to 100,000 IVIs will be administered annually in their service by 2029 alone [2]. Therefore, the need for an efficient, high-volume injection service is critical to keep pace with demand, although this may be tempered by longer acting drugs.
The Stanley Eye Unit (SEU) first introduced nurse injectors in 2016, after a standard operating procedure (SOP) for training was approved by the Betsi Cadwaladr Health Board. The SOP consisted of several theory sessions with an examination, a wet lab session, an external course, followed by a period of supervised practical sessions. Once 100 satisfactory IVIs are performed supervised, the AHP is signed off to perform IVIs autonomously.
As part of the initial SOP design, the decision was made to use an injection assistant device rather than perform the injections freehand. This was to give confidence to the injecting AHP that the distance from the limbus, the injection angle and injection depth were fixed, reducing the chance of iatrogenic injury.
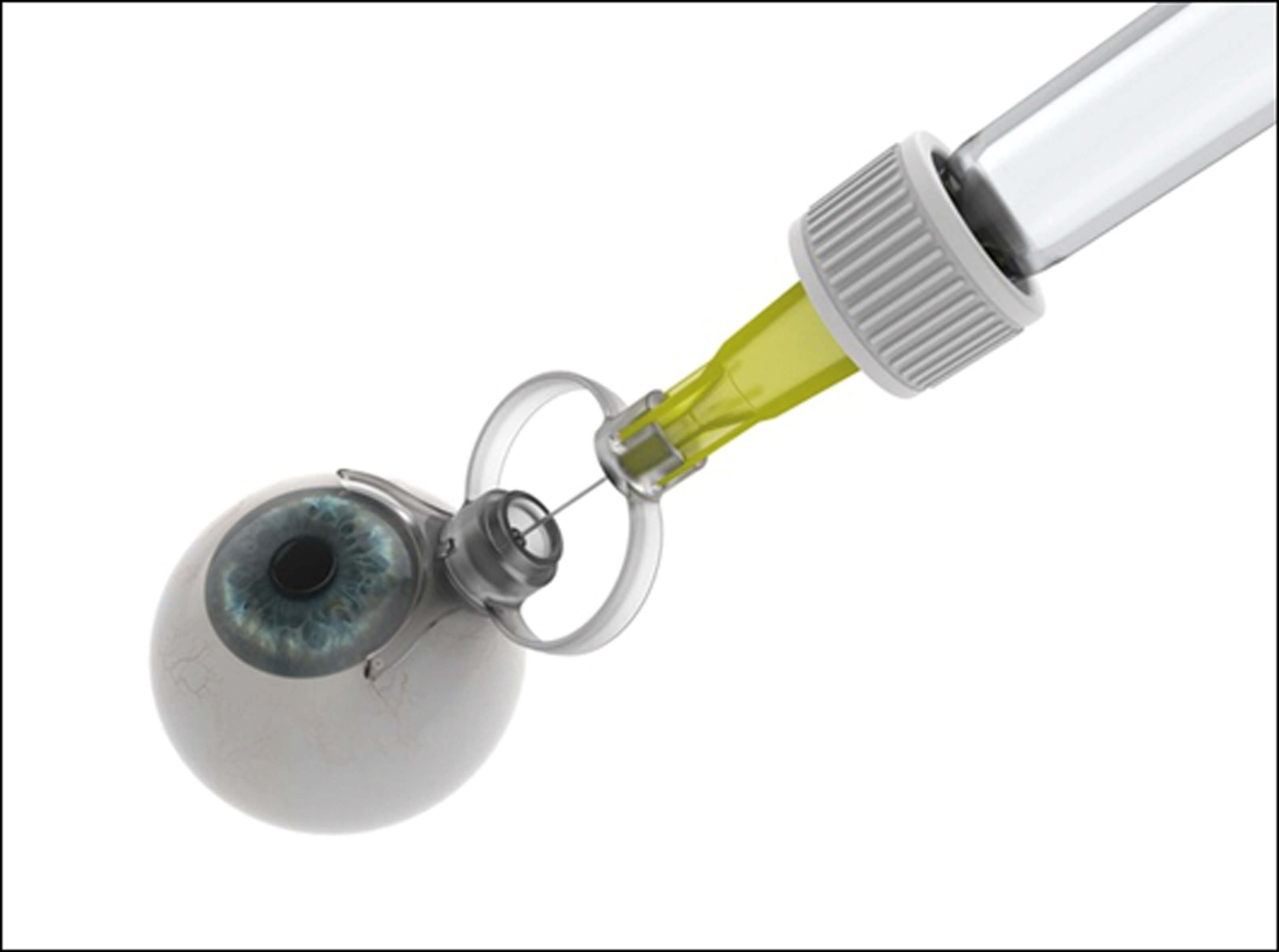
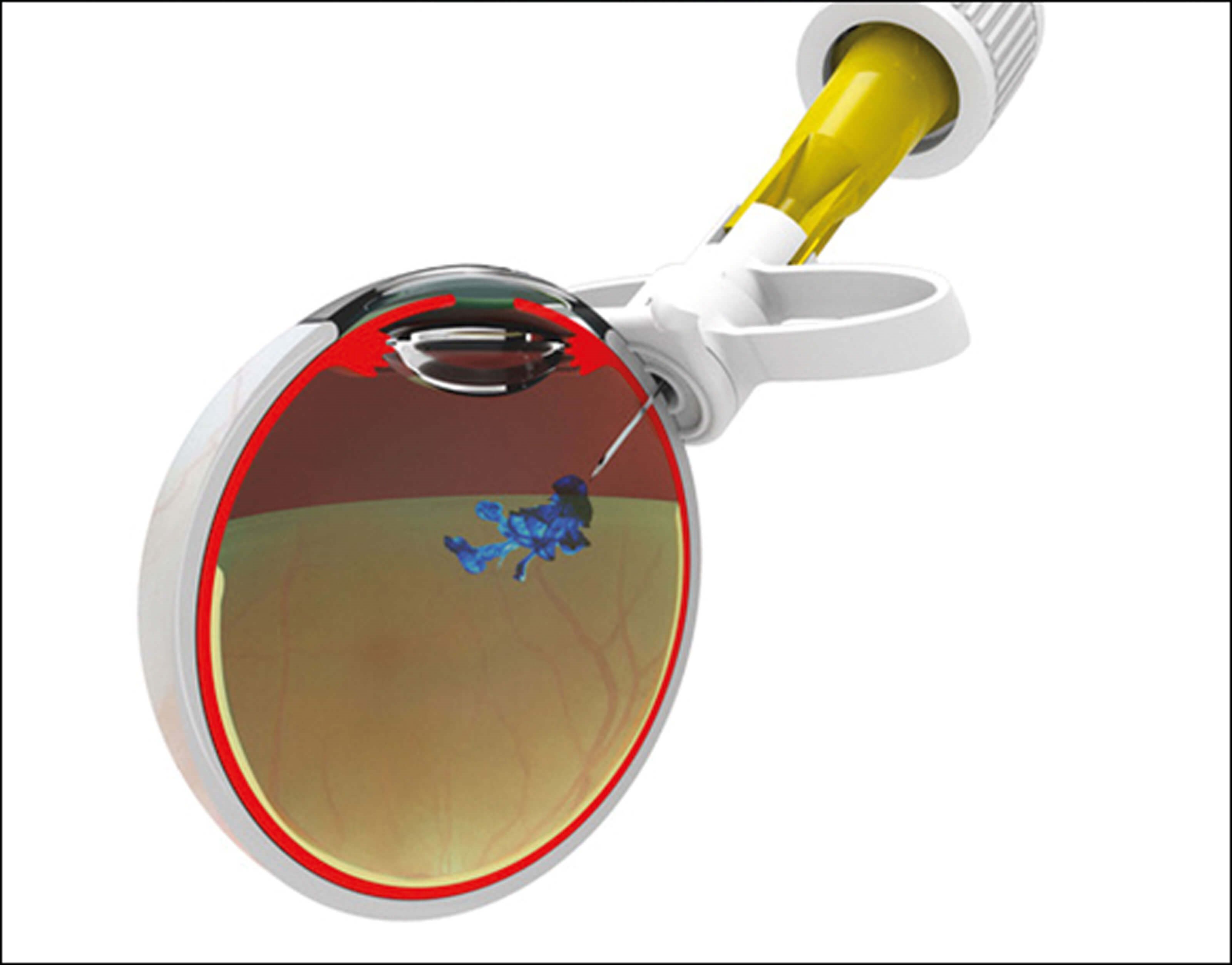
Figure 1. Sp.eye device in the ready to inject position (top)
and the injection delivery position (bottom).
About the Sp.eye
The Sp.eye injection assistant device is a ‘C’ shaped device that curves around the limbus with a built in 30G needle (see Figure 1). The Sp.eye is attached to the syringe (push-fit or luer-lock), primed and lined up with the limbus. Once in place, the injection is given at 4mm from the limbus in one single fluid movement, at an angle of 90° and depth of 6mm. It can be made sharp-safe at the end of the procedure. A video of the device in use can be found by following the QR code at the end of this article. The SEU chose to use the Sp.eye device largely based on its usability, patient eligibility and cost.
Some injection assistant devices have contraindications to their use, such as in the setting of a trabeculectomy or corneal graft. The Sp.eye device has no contraindications and therefore can be used in these settings. Deep set eyes and patients with small palpebral apertures are not more challenging because of the small size of the Sp.eye device. Additionally, the needle is not exposed during the preparation / alignment part of the injection, and this reduces the chance of inadvertent contact with the lashes. The enclosed needle design also has the benefit of not being at risk of blunting compared to assistant devices that require passing the needle down a narrow shaft. The Sp.eye device can be used with or without a speculum, depending on the injector’s preference.
The costs of Sp.eye and other alternatives were compared by our unit and at the time of purchasing, Sp.eye was cheaper to use per eye. The SEU chose to use the Sp.eye device with a speculum, but the cost saving remained significant.
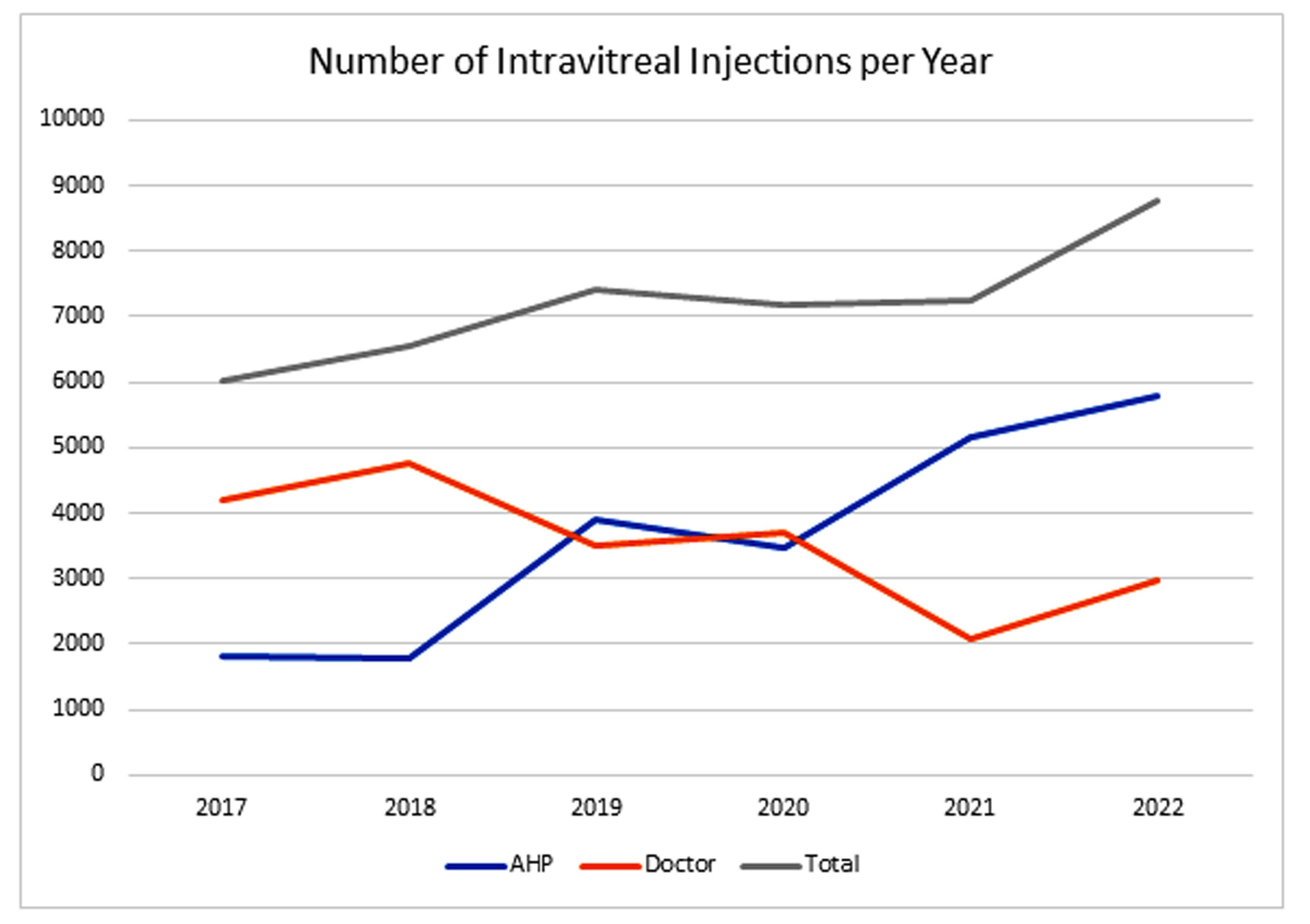
Figure 2: Number of injections per year administered by AHP, doctors and the combined total.
Over the last seven years, 13 AHPs have been trained to deliver IVIs, all using the Sp.eye device. The number of injections performed has continued to rise to keep pace with demand (see Figure 2). Many of the AHPs were redeployed during the Covid-19 pandemic and hence injection numbers dipped in 2020. Training up lots of AHPs has the benefit of spreading the injection load, and backfilling unexpected absences is easier.
The total number of IVIs given by AHPs in the SEU is in excess of 20,000, all using the Sp.eye device. The majority of the doctors who inject use the Sp.eye device, too. The injection lists are efficient with a typical session being around 18 eyes for injection.
Safety
Serious adverse events (SAE) such as infectious endophthalmitis, lens strike and retinal detachment following intravitreal injection are kept in an SAE log. During the last seven years, there have been no cases of lens strike or retinal detachment linked to an IVI. Over this period, eight cases of endophthalmitis have been linked to an injection procedure, 50% being with the use of an Sp.eye device and the other 50% being delivered freehand. The overall rate of infectious endophthalmitis at SEU is 0.02% over the seven-year period. This is within the reported rate of endophthalmitis, ranging from 0.02% to 0.05% [3,4].
The endophthalmitis rate was lower in the AHP injectors (0.01%) than the doctor injectors (0.02%), supporting the evidence base that AHPs can deliver IVIs safely [5,6]. It is known that patient preference for doctor injectors over nurse injectors is low, which further supports the use of training AHP to perform intravitreal injections [7]. We sought the opinion of an AHP injector who uses the Sp.eye device regularly:
“I first trained to perform IVT Injections in 2019 and our department at Abergele Hospital was using the Sp.eye device. The device is very simple to connect, check and prime prior to injection. The needle being within the device until injection ensures that it is sterile before entering the vitreous cavity.
“The ‘curved wings’ of the design make it very simple to place on the limbus so that you have total confidence that the injection is anatomically in the correct position (for phakic and pseudophakic patients) and the correct depth is always achieved. In my experience, it has a zero-failure rate – and the device is manually and visually checked before use (I check before and after priming)"
– Ken Dawes, Nurse practitioner, Abergele Hospital.
“In summary, it is clinically effective and efficient, with ease of use especially for trainees – and most importantly ensuring safety for patient care.”
Conclusion
The SEU has had extensive experience with the Sp.eye device and have demonstrated that it can be used safely by AHPs to deliver a high-volume injection service. It compares favourably in terms of price and lack of contraindications to its use.
References
1. Kirby G. College Statement on intra-ocular injections by non-medical health care professionals (HCPs), Royal college of Ophthalmologists, 2013.
2. Chopra R, Preston GC, Keenan TDL, et al. Intravitreal injections: past trends and future projections within a UK tertiary hospital. Eye 2022;36:1373–8.
3. Dossarps D, Bron AM, Koehrer P, et al. Endophthalmitis After Intravitreal Injections: Incidence, Presentation, Management, and Visual Outcome. Am J Ophthalmol 2015;160(1):17–25.
4. McCannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents: causative organisms and possible prevention strategies. Retina 2011;31(4):654–61.
5. DaCosta J, Hamilton R, Nago J, et al. Implementation of a nurse-delivered intravitreal injection service. Eye 2014;28:734–40.
6. Simcock P, Kingett B, Mann N, et al. A safety audit of the first 10 000 intravitreal ranibizumab injections performed by nurse practitioners. Eye 2014;28:1161–4.
7. Baxter JM, Fotheringham AJ, Foss AJ. Determining patient preferences in the management of neovascular age-related macular degeneration: a conjoint analysis. Eye 2016;30(5):698–704.
A video of the device in use can be found by here https://youtu.be/daB-zteP4xU
Declaration of competing interests: None declared.