Introduction
Intravitreal injection of neutralising anti-vascular endothelial growth factor (VEGF) antibody was licenced more than a decade ago, and over the years there has been proportionate increase in the number of intravitreal injections [1].
Injection of a therapeutic agent through the intravitreal route is generally safe, well tolerated and effective in stabilising vision in most and improving vision in some patients [2]. However, intravitreal injection therapy (IVT) carries a small but finite risk of post-intravitreal injection related bacterial endophthalmitis. Post-IVT related endophthalmitis is a manageable but serious complication and could lead to permanent visual morbidity. Therefore, it is paramount that measures should be taken to reduce the potential risk of this complication and ensuring high-quality care is delivered.
A rising number of retinal disorders are now being treated using intravitreal injections and recently, apart from anti-VEGF molecules, other theraputic agents have been developed and licensed for intervitreal use. To address the needs of increasing demand for intravitreal injections, ophthalmic nurses are trained to deliver intravitreal injections and dedicated IVT clinics are introduced in medical retina services [3].
We present real-world data from Moorfields Eye Hospital, London, on the incidence of post-intravitreal injection related endophthalmitis. We also evaluated incidence rate of post-IVT endophthalmitis in relation to different antiseptic agents used (povidone iodine and chlorhexidine) and compared incidence rate at different injection sites (superotemporal and inferotemporal).
Material and methods
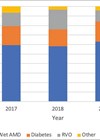
The most common indication for an intravitreal injection was neovascular age-related macular degeneration (nAMD) followed by diabetic macular oedema (DMO). We analysed two years of intravitreal injection data from 2014 to 2016 where a total of (n= 50,402) injections were delivered. Most of the intravitreal injections were delivered by trained ophthalmic nurses under the supervision of an ophthalmologist. No topical antibiotic was used pre, during or post-intravitreal injection procedure, and IVT procedure was postponed in patients with infective blepharitis. We evaluated:
(1) Incidence of post-intravitreal injection related endophthalmitis.
(2) Incidence of post-intravitreal injection related endophthalmitis with different antiseptic agent (5% povidone-iodine solution or aqueous chlorhexidine 0.02% as antiseptic agents). Most patients received 5% povidone-iodine as an antiseptic agent, however aqueous chlorhexidine 0.02% was only used in patients who were reported or suspected to be allergic to povidone-iodine.
(3) Incidence of endophthalmitis at different sites of injection (superotemporal and inferotemporal).
Results
During the two-year study period a total of 50,402 intravitreal injections procedures were performed by trained ophthalmic nurse injectors, and 12 cases of post-IVT related endophthalmitis were reported, therefore the incidence of endophthalmitis was one in 4000, 0.012% to 0.042%.
Five percent povidone-iodine was used in n=49,239 eyes and n=09 cases of endophthalmitis were reported with an estimated endophthalmitis incidence of one in 5000 (0.008% to 0.034%). Aqueous chlorhexidine 0.02% solution was used in a small proportion of patients n=1163 and reported cases of endophthalmitis in this cohort was n=03, therefore the incidence of endophthalmitis was one in 350 (0.05% to 0.75%). Overall, the incidence of endophthalmitis was lower in eyes receiving 5% povidone-iodine as compared to chlorhexidine (IRR (95 % CI) 0.07 (0.017 to 0.41, P=0.002)).
Comparing the rate of endophthalmitis at different sites of injection (superotemporal versus inferotemporal), out of n=50,402 injections performed during the study period, the majority of injections were performed at superotemporal site (n=31,705) with n=04 cases of endophthalmitis. Total number of injections carried out at inferotemporal site were n=18,697 and n=08 cases of endophthalmitis were reported in this group.
Therefore, in our cohort the incidence of endophthalmitis at superotemporal site was one in 8,000, 0.003% to 0.032%, as compared to inferotemporal site, one in 2,300, 0.018% to 0.084%. Overall, the incidence rate of endophthalmitis was lower at the superotemporal site (IRR (95% CI) 0.29 (0.064 to 1.099, P=0.44)).
Discussion
In current ophthalmic practice, intravitreal injection is the most commonly performed surgical procedure in medical retina service, and with increasing numbers of therapeutic agents being licenced for intravitreal intervention the workload is set to increase.
Intravitreal injection performed under sterile conditions is potentially safe, however there is a small risk of endophthalmitis and the reported incidence rate of endophthalmitis is around one in 2000 (0.025%-0.01%) [4-6], however, our real- world data showed even lower incidence of post- IVT endophthalmitis.
To cope with the increasing demand for intravitreal injections, in 2013, Moorfields Eye Hospital introduced nurse-delivered intravitreal injection service [3]. Band 7 ophthalmic nurses were trained in surgical techniques and supervised during the training process. Core surgical skills and efficacy of training was evaluated through regular audits, peer to peer led practice, feedback from patients and supervising ophthalmologists. In our current practice, the majority of injections are now delivered by trained ophthalmic nurses under the supervision of an ophthalmologist. So far, 54 nurse practitioners have been trained trust-wide, administering an average of 25,000 to 30,000 intravitreal injections each year [7]. Training and education are an integral component of any successful service. To ensure this, a robust and well-structured IVT injection training course is held by trust regularly to train healthcare professionals and ophthalmic nurses nationally and internationally [7]. Over the years we have observed an increasing level of confidence, both from ophthalmic professionals and from patients, and overall nurse- delivered intravitreal injection service is a success story.
To prevent endophthalmitis, prescription of topical antibiotics either pre or post-intravitreal injection, used to be a standard practice in most ophthalmic units in the developed world. However, recent scientific data showed that there is a weak clinical evidence to support, that topical antibiotics reduces the risk of post-intravitreal injection endophthalmitis. Also, there is clinical evidence to suggest that unnecessary use of antibiotic could lead to drug resistance [8,9]. In our cohort, the incidence of post-IVT related endophthalmitis without antibiotic cover was one in 4000 (0.023%) and we observed no increased incidence of endophthalmitis when injections were delivered without antibiotic cover. Therefore, in the absence of topical antibiotics, the use of an effective antiseptic agent is crucial to minimise the potential risk of infective endophthalmitis. The routine use of povidone-iodine as topical antiseptic agent remains the most effective and commonly practiced measure to minimise the risk of post-intravitreal injection related endophthalmitis [5,10,11].
Povidone-iodine has potent antiseptic properties and application of iodine containing antiseptic has been widely used in the medical field for almost two centuries, hence it is considered as the gold standard for topical antisepsis [12-14].
Unlike other antiseptic agents, povidone-iodine has an effective broad-spectrum anti- microbicidal activity against bacteria, viruses, fungi, spores, protozoa, and amoebic cysts [14-16]. Povidone-iodine bactericidal activity also covers two of the most commonly cultured organisms (coagulase-negative staphylococci and viridans group streptococci) isolated in cases with post-intravitreal injection endophthalmitis [17]. Unlike antibiotics, there have been no reports of resistance to povidone-iodine’s bactericidal activity [18,19].
Iodine is an integral part of human physiology; therefore it does not induce an allergic reaction; however, a small number of patients are reported to be allergic to povidone-iodine, which is mainly due to the povidone carrier molecule of the povidone-iodine compound [20].
There is a grey area between iodine allergy and iodine sensitivity; allergy is an immune mediated reaction manifesting as urticaria and contact dermatitis, whilst sensitivity is mainly due to irritant component of povidone in the povidone-iodine compound and can present as localised redness, foreign body sensation or epiphora. Some patients only demonstrate sensitivity to povidone-iodine, which is due to it’s irritant effect on contact with the ocular surface, and is proportional to the duration of exposure [21]. Therefore, in patients who are sensitive (not allergic) to iodine, 5% povidone-iodine can still be used as an antiseptic agent, however, through irrigation with normal saline should be practiced following IVT injection to minimise post-injection irritation and discomfort.
For patients who are reported to have an allergic reaction to povidone-iodine, an alternative antiseptic agent like aqueous chlorhexidine can be used. During the study period, 0.02% aqueous Chlorhexidine was used, however a stronger concentration (0.05% or 0.1%) of aqueous chlorhexidine can be used as an alternative antiseptic agent to minimise potential risk of endophthalmitis [23]. In view of this study’s findings, a higher concentration of chlorhexidine (0.05% aqueous chlorhexidine) is now being used in our trust as an alternate antiseptic agent.
In our cohort, some patients who developed endophthalmitis with chlorhexidine (as they were suspected to be allergic to povidone-iodine) later on, after successful management of endophthalmitis, were switched back to povidone-iodine, and no adverse effects they reported in those patients.
Therefore, in patients with a history of potential povidone-iodine related allergy, a detailed consultation with an ophthalmologist is crucial, prior to consent for the procedure. During initial consultation, we should try to differentiate between povidone-iodine related allergy and sensitivity, and if in doubt patients can be referred to local the dermatology department for repeat open application tests (ROATs). Anaphylaxis (a rare form of allergy) is a systematic antibody-mediated hypersensitivity reaction (type I hypersensitivity reaction); and there are no reported cases of anaphylaxis related to topical use of ophthalmic povidone-iodine [22].
In our study, a significantly lower incidence rate of endophthalmitis (10-fold lower) was noted in eyes treated with 5% povidone-iodine when used an antiseptic agent. Therefore, it is vital to counsel and educate patients prior to intravitreal injection procedure on potential efficacy of different antiseptic agents. In light of this study, we developed an 'iodine-patient education information leaflet', describing important findings of this study.
Ophthalmologists and ophthalmic nurses are trained to deliver intravitreal injections at superotemporal or inferotemporal sites comfortably. However, site of injection varies depending on injector’s preference, accessibility, and previous glaucoma surgery. The superotemporal site offers greater exposure, easy accessibility and makes it relatively easy to manage retinal tear complications with pneumatic retinopexy, though the incidence of retinal tear and retinal detachment is a rare complication with intravitreal injections [24].
In our cohort, a slightly higher rate of endophthalmitis was reported in eyes receiving injections at the inferotemporal site, and this could be secondary to direct contact of the lower lid with the injection site (risk is higher with underlying lid infection like blepharitis), pooling of tear film and potential exposure to air [25,26].
In this study, although the incidence rate of endophthalmitis was higher at the inferotemporal site however, there was no statistically significant difference noted when compared to endophthalmitis rate observed at the superotemporal site. Therefore, further studies are underway to evaluate this aspect of post-IVT endophthalmitis.
Conclusion
In current ophthalmic practice, regular dosing of intravitreal therapeutic agents is vital to stabilise and maintain vision, therefore we must ensure that our intravitreal therapeutic delivery service is safe, effective, and responsive to patient’s needs. With ageing population and increasing diabetes, the demand for intravitreal injection therapy is set to increase, therefore, shared- care model is the way forward. Our, nurse - led IVT service is safe, effective and has evolved on evidence-based medicine.
Intravitreal injection-related endophthalmitis is a rare but vision-threating complication, therefore choice of appropriate and effective antiseptic agent, good aseptic technique and detailed pre-injection consultation is necessary to minimise the potential risk of post-intravitreal endophthalmitis.
References
1. NICE technology appraisal guidance 155; issued August 2008, modified May 2012. Ranibizumab and pegaptanib for the treatment of age-related macular degeneration.
http://www.nice.org.uk/TA155
Last accessed December 2022.
2. Liew G, Lee AY, Zarranz-Ventura J, et al. The UK Neovascular AMD Database Report 3: inter-centre variation in visual acuity outcomes and establishing real-world measures of care. Eye 2016;30(11):1462-8.
3. DaCosta J, Hamilton R, Nago J, et al. Implementation of a nurse-delivered intravitreal injection service. Eye 2014;28(6):734-40.
4. Lyall DAM, Tey A, Foot B, et al. Post-intravitreal anti-VEGF endophthalmitis in the United Kingdom: incidence, features, risk factors, and outcomes. Eye (Lond) 2012;26(12):1517-26.
5. Fileta JB, Scott IU, Flynn Jr HW. Meta-analysis of infectious endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmic Surg Lasers Imaging Retina 2014;45(2):143-9.
6. Moshfeghi AA, Rosenfeld PJ, Flynn Jr HW, et al. Endophthalmitis after intravitreal anti-VEGF antagonists: a six-year experience at a university referral centre. Retina 2011;31(4):662-8.
7. Mapanim A, Hamilton R, Nago J, et al. Celebrating eight years of the nurse-led intravitreal injection service. Eye News 2021;27(5):16-20.
8. Li AL, Wykoff CC, Wang R, et al. Endophthalmitis after intravitreal injection: role of prophylactic topical ophthalmic antibiotics. Retina 2016;36(7):1349-56.
9. Haas W, Pillar CM, Torres M, et al. Monitoring antibiotic resistance in ocular microorganisms: results from the Antibiotic Resistance Monitoring in Ocular microorganisms (ARMOR) 2009 surveillance study. Am J Ophthalmol 2011;152(4):567-74.
10. Green-Simms AE, Ekdawi NS, Bakri SJ. Survey of intravitreal injection techniques among retinal specialists in the United States. Am J Ophthalmol 2011;151(2):329-32.
11. Merani R, Hunyor AP. Endophthalmitis following intravitreal anti-vascular endothelial growth factor (VEGF) injection: a comprehensive review. Int J Retina Vitreous 2015;1:9.
12. Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology 1991;98(12):1769-75.
13. Apt L, Isenberg SJ, Yoshimori R, Spierer A. Outpatient topical use of povidone-iodine in preparing the eye for surgery. Ophthalmology 1989;96(3):289-92.
14. Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, et al. Povidone iodine in wound healing: A review of current concepts and practices. Int J Surg 2017;44:260-8.
15. Kumar J, Jayachandran KE. Reddy HK, et al. Application of broad-spectrum antiseptic povidone iodine as powerful action: a review. J Pharm Sci Technol 2009;1(2):48-58.
16. World Health Organization (2016), WHO; Global Guidelines for the Prevention of Surgical Site Infection.
https://apps.who.int/iris/bitstream/handle/
10665/250680/9789241549882-eng.pdf
17. Busch C, Iglicki M, Okada M, et al. Causative pathogens of endophthalmitis after intravitreal anti-VEGF injection: an international multicentred study. Ophthalmologica 2019;241(4):211-9.
18. Lachapelle JM, Castel O, Casado AF, et al. Antiseptics in the era of bacterial resistance: a focus on povidone iodine. Clin Pract 2013;10(5):579-92.
19. Kunisada T, Yamada K, Oda S, Hara O. Investigation on the efficacy of povidone-iodine against antiseptic-resistant species. Dermatology 1997;195(Suppl 2):14-8.
20. Wykoff CC, Flynn Jr HW, Han DP. Allergy to povidone-iodine and cephalosporins: the clinical dilemma in ophthalmic use. Am J Ophthalmol 2011;151(1):4-6.
21. Merani R, Hunyor AP. Endophthalmitis after intravitreal injections in patients with self-reported iodine allergy. Am J Ophthalmol 2017;176:256-7.
22. Krohne TU, Allam JP, Novak N, Holz FG. “Iodine allergy”: a medical myth with risks for the ophthalmological patient. Ophthalmologe 2016;113(12):1023-8.
23. Merani R, McPherson ZE, Luckie AP, et al. Aqueous chlorhexidine for intravitreal injection antisepsis: a case series and review of the literature. Ophthalmology 2016;123(12):2588-94.
24. Severn PS, Hamilton R. The incidence of serious complications associated with intravitreal therapy in a quaternary ARMD service (2008–2014). Eye (Lond) 2015;29(1):150.
25. Shrier EM, Raevis J, Li J, et al. Safety of superior-temporal intravitreal injections using the cotton-tip applicator lid retraction technique. J Ocular Biol 2017;5(1):4.
26. Jonna G, Roth DB, Fine HF, et al. Inferior intravitreal injection site associated with higher incidence of post-injection endophthalmitis. Int Eye Sci 2015;15(5):750-4.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME