The ocular surface (OS) is an anatomical and functional unit made of the tear film, the conjunctival, limbal and corneal epithelium, the lacrimal, mucous and meibomian glands and the lids and blink reflex. The tear film is composed of a basal layer of mucin derived from the goblet cells and the underlying epithelium. Mucin makes the hydrophobic epithelial surface into a hydrophilic surface and spreads in a reducing gradient into the overlying aqueous component derived from the lacrimal gland.
The surface of the tear film is a thin layer of lipid secreted by the Meibomian gland that retards evaporative loss of the tears. The tear film provides the final optical polish to the corneal surface for accurate focusing of transmitted light. The corneal epithelium is constantly shed and replenished by the proliferation and migration of cells from the basal layer and from stem cells located at the limbus. The inter-palisade rete ridges of the palisades of Vogt and the limbal epithelial crypts (Figure 1A) that extend into the underlying stroma from some inter-palisades ridges along the circumference of the cornea, are repositories of stem cells. The conjunctival blood vessels become specifically organised at the limbus and contribute to the microenvironment of the stem cell niche; eventually terminating as the peripheral vascular arcade of the otherwise avascular cornea.

Figure 1: A) A limbal epithelial crypt, a putative repository of limbal stem cells, is seen extending into the limbal stroma from the posterior end of an interpalisade (of Vogt) rete ridge. B) A sub Bowman’s stromal nerve (arrowhead) is seen to end in a terminal bulb (arrow) after penetrating through the Bowman’s membrane. Neurites of the sub-basal plexus are seen arising from the bulb (From the author’s own publication from Br J Ophthalmol 2010;94:784-9).
A very important but less emphasised aspect of the ocular surface is its innervation from the ophthalmic division of the fifth cranial nerve. Approximately 11 nerve trunks per quadrant of the cornea, enter at the limbs and divide dichotomously as they approach the sub-Bowman’s zone and form a plexus from which nerve fibres pass anteriorly through the Bowman’s membrane and terminate in terminal bulbs. From these bulbs multiple neurites arise and divide and subdivide and inter-connect to form the sub-basal plexus. Terminal nerve endings from the sub-basal plexus pass vertically between epithelial cells and terminate both inter and intra cellularly. The nerves primarily serve a vasomotor function in the peri-limbal zone, causing ciliary congestion following corneal insult, and a sensory and trophic function in the cornea (Figure 1B).

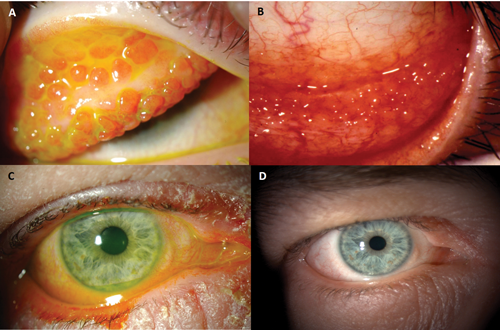
Figure 2: A) Fine punctate keratitis affecting the lower third of the cornea in a patient with dry eyes. B) Coarse punctate keratitis affecting the upper cornea of a patient with dry eyes associated with rheumatoid arthritis. C) Filaments with mucus coating in a patient with rheumatoid arthritis related dry eyes. D) Central coarse punctate keratitis in a patient with post-Lasik ‘dry eye’. All eyes are stained with 2% fluorescein dye.
‘Dry eyes’ is the commonest OS disease related to tear film quantity, composition and distribution. Dry, gritty, irritable, sensitive, burning eyes are some of the expressions used by patients to describe their symptoms. Fine or coarse punctate keratitis, filaments (Figure 2A, B, C), narrow tear meniscus, rapid tear break-up time and low Schirmer’s test values are important signs. Superficial punctate keratitis (SPK) usually manifests in the lower third of the cornea and conjunctiva (Figure 2A). However, in dry eyes associated with refractive surgery SPK is more central and most probably related to nerve injury (Figure 2D). Signs of blepharitis and associated local and systemic disease are often present. Symptoms and signs, however, do not often correlate. Management tips include the following:
- Symptoms worse than signs (or no signs): treat.
- Signs mild but no symptoms: can watch but beware of reduced corneal sensations.
Signs significant but no symptoms: treat (can be indicative of neurotrophic disease). - Beware of concomitant medication especially with preservatives.
- Patients with adequate reflex tearing are unlikely to develop serious problems.
- Patients who do not respond to conventional medication and show evidence of progressive worsening could have more sinister underlying disease such as ocular cicatricial pemphigoid and referral to a specialist should be considered. Such conditions are, however, rare.
Artificial tear drops are the mainstay in symptom relief. Anti-inflammatory agents especially steroids and cyclosporine are useful in controlling the inflammatory element of dry eyes. Punctal plugs, lid hygiene, dry and moist heat to the lids with devices such as the ‘Eye Bag’ and ‘Blephasteam’ and protective goggles are useful adjuncts. Control of environment with humidifiers at home and avoidance of working in the vicinity of electronic devices (computers) where the exhaust fan constantly spews out hot air, which enhances tear evaporation; should be considered. Newer approaches using mucin stimulators and hormone therapy are around the corner.
Figure 3: A) Large papillae in the upper palpebral conjunctiva. There is dense scarring in between papillae. B) Follicular reaction in the lower palpebral conjunctiva. Both papillae and follicles are non-specific responses to chronic irritation. C) Lid excoriation and ulceration with conjunctival hyperaemia in a patient with atopic keratoconjunctivitis. D) The same eye after a month treatment with protopic 0.03% immunosuppressive skin cream.
Allergic and immune disease
OS diseases can be inflammatory, degenerative, dystrophic, neoplastic or traumatic. Inflammatory (infective or non-infective) and trauma are common. Acute allergic conjunctivitis (AAC), seasonal allergic conjunctivitis (SAC), perineal allergic conjunctivitis (PAC), vernal keratoconjunctivitis (VKC), atopic keratoconjunctivitis (AKC) and giant papillary conjunctivitis (GPC) are the common allergic eye disease that primarily affect the OS. Of these, only VKC and AKC affect the cornea and can be sight-threatening. Hyperaemia of the fornices and palpebral conjunctiva, papillae and follicles are common signs (Figures 3A & B).
Papillae have a central core of blood vessels covered with hypertrophic epithelium and are usually seen in the upper palpebral conjunctiva. Follicles are collections of lymphocytes and are usually seen in the inferior fornix. Both are non-specific responses to chronic inflammation. AAC, SAC and PAC can be managed with non-steroid anti-inflammatory agents, antihistamines and mast cell stabiliser eye drops and generally do not need steroids. VKC and AKC on the other hand often require steroid and other immunosuppressive topical medication such as cyclosporine and tacrolimus (protopic) (Figures 3C & D).

Figure 4: Stage 2 ocular cicatricial pemphigoid (OCP) with fornecial shortening (short arrows) and symblepharon (long arrows). In the early stages OCP is often treated as dry eyes.
Ocular cicatricial pemphigoid (OCP) is an example of immune mediated chronic progressive cicatrising disease of the OS which, if unchecked leads to loss of sight. Immune complex deposition occurs on basement membranes of mucosal surfaces (conjunctiva, oral mucosa), and attracts inflammatory cells which release enzymes resulting in tissue damage; and growth factors that stimulate fibroblast proliferation and scarring. Concomitant affection of the oral mucosa occurs in 85% of cases and of the skin in 25%. Over the age of 60 years, female to male ratio is 2:1. The key is in maintaining an index of suspicion and early diagnosis. In its early stages OS inflammation is the only presentation and is often diagnosed as dry eyes or conjunctivitis. When scarring commences, effacement of the plica and shortening of the fornix (Figure 4) are early clues for referral to specialist centres. Treatment is complex and even more so when cicatrisation is excessive.
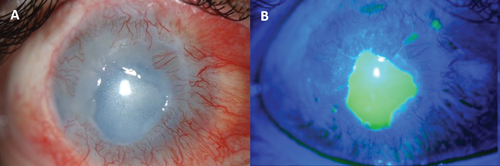
Figure 5: A) A persistent corneal epithelial defect (PED) with some stromal melting following chemical injury. The cornea also shows vascularisation. B) The extent of the PED is highlighted by fluorescein staining.
Persistent corneal epithelial defects
Corneal epithelial defects that do not heal or heal and breakdown repeatedly are termed ‘persistent epithelial defects’ (PED) (Figure 5). PED can result from a number of causes:
- Neurotrophic such as after fifth cranial nerve damage by surgery or trauma and herpes zoster or simplex keratitis.
- Limbal stem cell deficiency with can be due to chemical burns, chronic inflammation such as Stevens John Syndrome and ocular cicatricial pemphigoid or infections such as trachoma.
- Infections due to bacteria, acanthamoeba and herpes virus.
- Other conditions, e.g. diabetes, vitamin A deficiency, chronic use of drugs (nonsteroidals and steroids) and their preservatives (benzylkonium) and exposure as after facial palsy. PED can lead to stromal thinning and melting, scarring and even corneal perforation. Treatment is usually followed in a step ladder manner starting with simple medical measures to surgical intervention.
The underlying cause has to be identified and addressed. Topical lubricants including preservative free artificial tear drops, gels and ointments are useful. Sodium hyaluronate offers both lubrication and facilitates the epithelial cell migration and possibly adhesion. Autologous serum (20% to 100%) or plasma eye drops are quite effective. Some have expressed caution in using these drops in patients with autoimmune conditions, where circulating antibodies in the patients’ blood are part of the pathogenesis. Nerve growth factor and Substance P peptide offer considerable promise and are undergoing clinical trials. Regenerating agents (Cacicol) (poly(carboxymethylglucose sulfate) which is an analogue of heparan sulfate (extracellular matrix component) also known as matrix therapy, restore the collagen milieu and replenish binding sites for growth factors and other cytokines that render the substrate conducive to epithelial cell attachment. A bandage contact lens can be effective in promoting healing by protecting the healing epithelium.
Interventional measures essentially involve closure of the eye with simple measures such as a pad and bandage or ‘Frost sutures’ wherein a mattress suture is passed through the upper or lower lid close to the margin and the thread used as a handle to pull the eye lid to close the eye. The suture is taped to the cheek or forehead. Induced ptosis with botulinum toxin also achieves the same purpose. These allow the eye to be opened for instillation of drops. Central or lateral tarsorrhaphy (temporary or permanent) is the gold standard intervention for PED. Amniotic membrane has been used very effectively, both as a patch (temporary cover and then removed) or graft (epithelium grows on the membrane which becomes incorporated in the cornea).
Recurrent corneal erosion syndrome Recurrent corneal erosion syndrome refers to a condition characterised by repeated episodes of spontaneous breakdown of the corneal epithelium. It is characterised by severe pain, usually first thing on waking and can be very debilitating. Previous corneal injury with organic matter such as paper, fingernail, leaf or twigs; corneal dystrophies especially map dot finger print dystrophy and granular and lattice stromal dystrophies are common underlying conditions. It can be idiopathic.

Figure 6: A) Recurrent corneal erosion (RCE) with positive (green) and negative (blue) fluorescein staining. The image was taken soon after an episode of erosion. B) Fine intraepithelial cysts seen in a patient who suffered from RCE. These are visible on the slit-lamp and can be missed if not specifically looked for.
Fluorescein staining helps in establishing the diagnosis. Affected areas of the cornea appear as areas of negative or positive staining (Figure 6A). Features of associated dystrophy may also be seen. Careful examination is important as at times the only evidence may be the presence of fine intra-epithelial microcysts (Figure 6B).
Treatment in mild cases is with lubricant eye drops and especially ointment at bedtime to prevent the palpebral conjunctiva ‘sticking’ to the corneal surface. If this does not provide relief, a bandage contact lens (BCL) may be tried together with preservative artificial tear drops. The BCL needs to be changed every two to three weeks. At the end of a three-month trial it can be removed and the eye assessed for symptoms and signs. Lubricant drops should be continued. If the symptoms settle for a prolonged period of time and then recur, the BCL can be inserted again. When a BCL is used in the presence of an epithelial defect, prophylactic antibiotic, e.g. Chloramphenicol minims, should also be used.
If symptoms do not settle or recur soon after removal, an interventional option will be needed. Interventional options include phototherapeutic keratectomy (PTK), alcohol delamination of the corneal epithelium, diamond burr polishing and anterior stromal puncture. Anterior stromal puncture can lead to small scars and should be avoided if the pathology is in the visual axis. Outcomes of the other three options are very similar PTK is more expensive and also ablates a few microns of the corneal stroma, unlike the other two.
Limbal stem cell deficiency
A number of conditions can damage the limbus and lead to limbal stem cell deficiency (LSCD). Aniridia is the commonest congenital condition. Thermal and chemical injury, chronic inflammation due to trachoma, ocular cicatricial pemphigoid, Stevens Johnson syndrome, prolonged contact lens wear, preservative toxicity, ocular surface malignancy and exposure to ultraviolet and ionising radiation are the important acquired causes. Manifestations of LSCD can be minor with conjunctivalisation of the cornea at the periphery or in the form of columns of abnormal cells streaming in from the peri-limbal conjunctiva on to the cornea, alternating with similar columns of normal corneal cells – ‘columnar keratopathy’. Subtle conjunctivalisation of the cornea can be seen with late fluorescein staining.
Fluorescein staining is a very simple and important test in the examination of the OS. After explaining to the patient the nature of the examination, a drop of 2% fluorescein is instilled in the conjunctival sac and the patient asked to blink a few times. This ensures an even spread of the dye across the surface. One or two drops of normal saline are then instilled in the conjunctival sac and the patient asked to blink a few times again. Excess dye that may flow out is gently dabbed with a tissue. Immediate examination with the cobalt blue light will only show a ‘sea of green’. After a few seconds, the tear film breaks up and patterns on the cornea, related to late staining of cells, can be visualised such as conjunctivalisation of the cornea, pseudodendrites and coarse punctate lesions of epithelial acanthamobea keratitis, fine and coarse punctate keratitis of dry eyes, vortex or whorls staining of ‘hurricane keratitis’ and negative and positive staining of subtle changes of recurrent corneal erosions.

Figure 7: A) Early limbal stem cell deficiency (LSCD). Alternating columns of fluorescein stained cells and unstained cells (columnar keratopathy) seen in the upper part of the cornea. There is a triangular epithelial defect as well. B) Early LSCD. The visual axis is covered by a sheet of fluorescein stained epithelial cells (conjunctivalisation of the cornea) arising from the upper limbus. Both A and B can be treated with sequential sector conjunctival epitheliectomy. C) Total LSCD following chronic inflammation. Superficial and deep vascularisation is seen all around the cornea. The central cornea is scarred and has a persistent epithelial defect (D).
Conjunctivalisation of cornea is considered to be the hallmark of LSCD. This can be confirmed by impression cytology or in vivo confocal microscopy to demonstrate goblet cells. Recurrent erosions and filaments, corneal vascularisation, fibrovascular panus, non-healing epithelial defects, corneal scarring, perforation or calcification are other manifestations (Figure 7A-D).
Following acute injury (e.g. chemical injury) or disease, when the limbus is partially affected, LSCD can be prevented by brushing off the advancing conjunctival cells and allowing the corneal surface to be re-epithelised from the surviving limbal epithelium. This procedure is termed sequential sector conjunctival epitheliectomy (SSCE). SSCE can also be performed when patients present with conjunctivalisation of the cornea, wherein the abnormal late staining epithelium is brushed off repeatedly if necessary until the surface heals with normal limbus derived epithelial cells. In cases of unilateral total LSCD autologous limbal transplantation from the other normal eye is the preferred choice. Tissue from the normal eye can be directly transplanted to the affected eye after the surface has been cleaned by removing all fibrovascular panus tissue and conjunctival epithelium.
“Conjuncti-valisation of cornea is considered to be the hallmark of LSCD.”
The procedures are termed auto-limbal grafts, conjunctival limbal autografts and simple limbal epithelial transplantation. In cases of bilateral total LSCD allo-limbal grafts from a living related donor or cadaver donor are used for transplantation. The former can be tissue matched to reduce risk of rejection. The amniotic membrane is often used as an adjunct either to provide a suitable bed for the cells to grow on or to cover the transplant and afford protection (as a biological bandage) or to redirect conjunctival epithelial cells and prevent them from mixing with the desired limbal transplant derived corneal epithelial cells (amnion assisted conjunctival epithelial re-direction).

Figure 8: A) Total unilateral limbal stem cell deficiency treated with autologous limbal transplant and amniotic membrane graft tacked in place with a running suture. B) The same eye as in (A) stained with fluorescein. The limbal explants at 12 and 6 o’clock positions do not stain and early epithelial cell growth can be seen from both explants. The amnion surface and the peri-corneal surface from which the conjunctiva has been recessed show fluorescein staining.
For both auto and allo grafts, limbus derived cells can first be expanded ex-vivo (in specialised laboratories accredited for the purpose) as sheets often on a suitable substrate like the amniotic membrane or sheets of fibrin and then transplanted directly on the affected recipient eye. Allo grafts always require systemic immunosuppression to prevent rejection of transplanted tissue. To contend with the issue of rejection, at times autologous conjunctival or oral mucosal cells are used as ex-vivo expanded sheets. The long-term outcome of auto limbal tissue transplants is the best, followed by that of living related tissues and then by cadaver tissue. Autologous conjunctival epithelium and oral mucosal cells do not reject but the visual outcome is not as good as that after use of limbus derived cells (Figure 8).
The author is part of the Fight for Sight Speaker Network. This article is based on his keynote address at 100% Optical 2016, held at the Excel Centre, London on 8 February 2016. The following meeting was held on 4-6 February 2017 at the Excel in London.

Further reading
-
BenEzra D (ed): Blepharitis and Conjunctivitis. Guidelines for diagnosis and treatment. Editorial Glosa: Barcelona; 2006.
-
Gomes JAP, Perez VL, Scorsetti DH (eds): Stem Cells in Ophthalmology. Jaypee Highlights: Panama City; 2013.
-
Dua HS, Said DG. Ocular surface: Functional anatomy, diagnosis and management. In ESASO course series: Cornea. Güell JL (ed). Basel: Karger; 2015:1-25.
Declaration of Competing Interests
The author has received professional fees (honoraria, travel expenses, research funds) from Thea, Nicox, Chroma, Allergan, Dompe, Bausch & Lomb and has shares in Glaxo Smithkline Beecham.
COMMENTS ARE WELCOME