Transparency is a vital attribute of the cornea, necessary in preserving healthy vision. Maintaining this requires the collective input of the various layers of the cornea. Mostly, the layers implicated in this are the corneal stroma and endothelium [1].
Our study conducted at the Manchester Royal Eye Hospital involved elucidating the relationship between corneal clarity and statin use in participants with healthy corneas. Before we explore this further, we must first appreciate how corneal clarity is established and maintained physiologically.
The stroma maintains corneal clarity through the action of keratocytes (corneal fibroblasts found mainly in the anterior stroma). Massoudi, et al. identify this action as producing type V collagen, fibril-associated collagen with interrupted triple helices (FACITs) and small leucine-rich proteoglycans (SLRPs) [2]. Numerous studies on this topic have theorised that collagen-proteoglycan interactions directly relate to corneal transparency [3]. In addition, Sivak, et al. suggest that the keratocytes also manufacture proteolytic enzymes known as matrix metalloproteinases to further facilitate stromal homeostasis by remodelling the corneal architecture [4]. Aside from these components, Gong, et al. suggest that crystallins are also implicated in preventing corneal opacity by supposedly reducing light scatter [5]. Previously considered to only be present within the crystalline lens, examples of these compounds include aldehyde dehydrogenase and trans-ketolase.
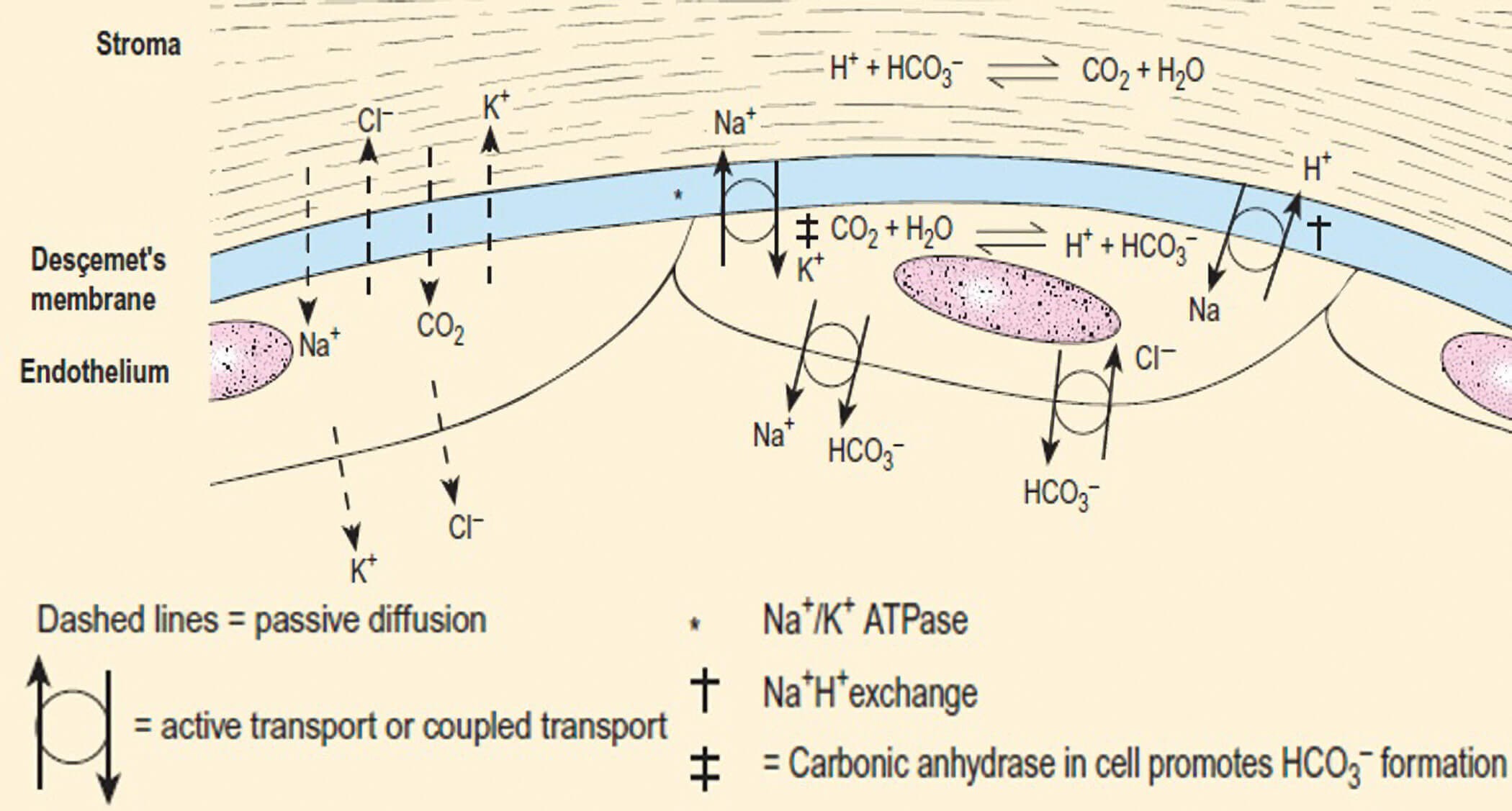
Figure 1: The corneal endothelial pump with ion transport mechanisms outlined (legend provided) [1].
The corneal endothelium has its own designated role in simultaneously safeguarding corneal clarity as well as maintaining the turgidity of the stroma. It undertakes these processes via the endothelial pump which alters the hydration status of the corneal proteoglycans (Figure 1). This involves ensuring that the binding of water molecules is unsaturated so that they may easily be shifted into the anterior chamber from the cornea via active transport [6]. In doing so, it makes use of an ATP-dependent pump-leak mechanism. This transports chloride (Cl-) ions using the cystic fibrosis transmembrane conductance regulator (CFTR) [1]. Alongside this energy-driven process, there is an electrogenic coupling or ‘anti-port’ system in place between sodium (Na+) and bicarbonate (HCO3‑) ions which utilises an Na+ / H+ exchanger protein as well as carbonic anhydrase to maintain the intracellular pH.
Simultaneously, a mechanism has been revealed involving the bulk transport of water and carbon dioxide (CO2) molecules between the corneal stroma and endothelium [6]. This occurs via independent water transporter channels, such as aquaporins, in the endothelial membrane to carry out this passive movement. This is the endothelial response system in relation to sudden alterations in stromal hydration as well as endothelial cell volume. Li, et al. highlighted a metabolic transport system for water molecules comprising the use of lactate ions and their high concentration gradient towards the anterior chamber using transcellular monocarboxylate co-transporters [6]. This expulsion of water to maintain a state of relative dehydration is referred to as ‘deturgescence’.
With regards to the role of statins, the primary corneal change which has been studied revolves around the integrity of the endothelial layer. Initially, studies by Satpathy, et al. highlighted the role of thrombin in the breakdown of the corneal endothelium barrier [7]. It carries this out via the process of phosphorylation carried out on myosin light chains (MLC) which are subunits of motor proteins involved in muscle contraction. This chemical change is mediated by the GTPase protein RhoA which plays a key role in cytoskeleton regulation. This causes tight junction dysfunction at the peri-junctional actomyosin ring (PAMR) of the endothelial cells. This leads to the barriers between the internal cellular contents and paracellular space being compromised [8]. Subsequent studies showed that statins inhibit phosphorylation, thus preventing the cascade of events leading to endothelial barrier breakdown.
Methods
To investigate the link between corneal clarity and statin use, the healthy corneas of 169 eyes from 85 participants aged 20–89 years old were observed using OCULUS Pentacam® devices at the University of Manchester and the Manchester Royal Eye Hospital. The data obtained included age, statin status and corneal densitometry values, a measure of corneal opacity scaled from 0–100. The densitometry values were taken from concentric corneal zones 0–2mm, 2–6mm, 6–10mm and 10–12mm, encompassing the corneoscleral limbus, divided into three layers of depth: anterior, central, and posterior cornea. The data was collected via a rotating Scheimpflug camera which received numerous slit-lamp beams during the 30-second process. It was then displayed in table form with associated three-dimensional imaging of the participant’s cornea (Figure 2) [9].
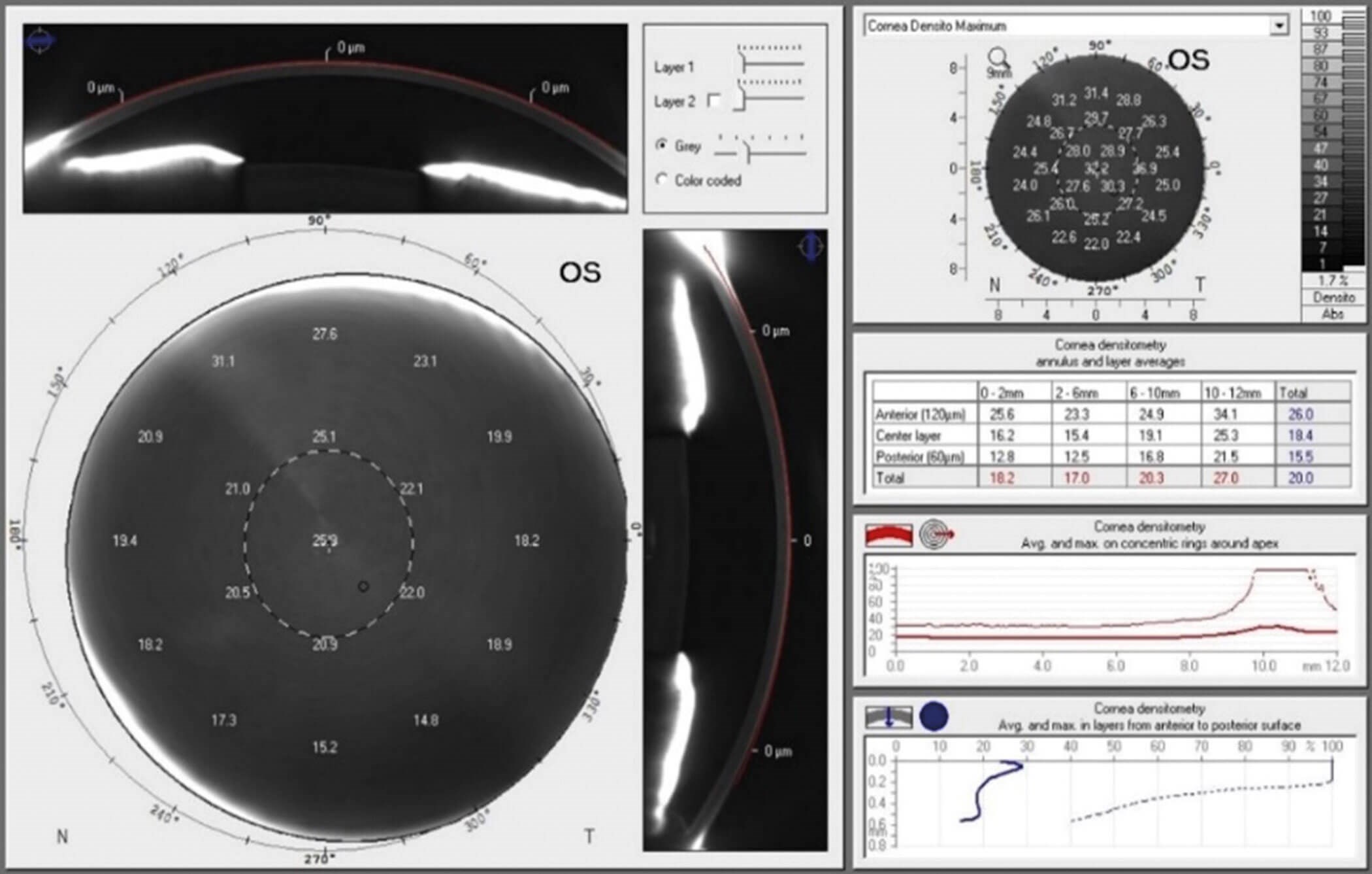
Figure 2: OCULUS Pentacam® topographical densitometry display [9].
Results
After the data from participants not within the correct age range and / or from those with existing corneal disease were excluded, 169 eyes with viable corneas from 85 participants were evaluated. Out of this cohort, 41 participants were found to be on statins whilst the remaining 44 were not prescribed the medication. As there were no statin users in the cohort under 50 years old, the analysis was confined to participants aged 50 to 89, with data divided into 50–59 years, 60–69 years, 70–79 years, and 80–89 years age groups.
In terms of the 50–59 years age group, the data showed a trend indicating that mean densitometry values increased from the 0–2mm to the 10–12mm concentric zones across all layers of corneal depth, ranging from 19.29 to 32.63 units. It also showed that mean densitometry values were greater, albeit slightly, for statin users in all but one zone as compared to the non-users. In addition, this mean difference was most clearly seen in the 6–10mm concentric zone of the anterior corneal layer. The statin users expressed 4.86 higher units of corneal densitometry compared to non-users on average. However, these results were statistically insignificant for the concentric zones across all levels of depth from 0–2mm to 10–12mm with p-values of 0.390, 0.583, 0.551, and 0.573.
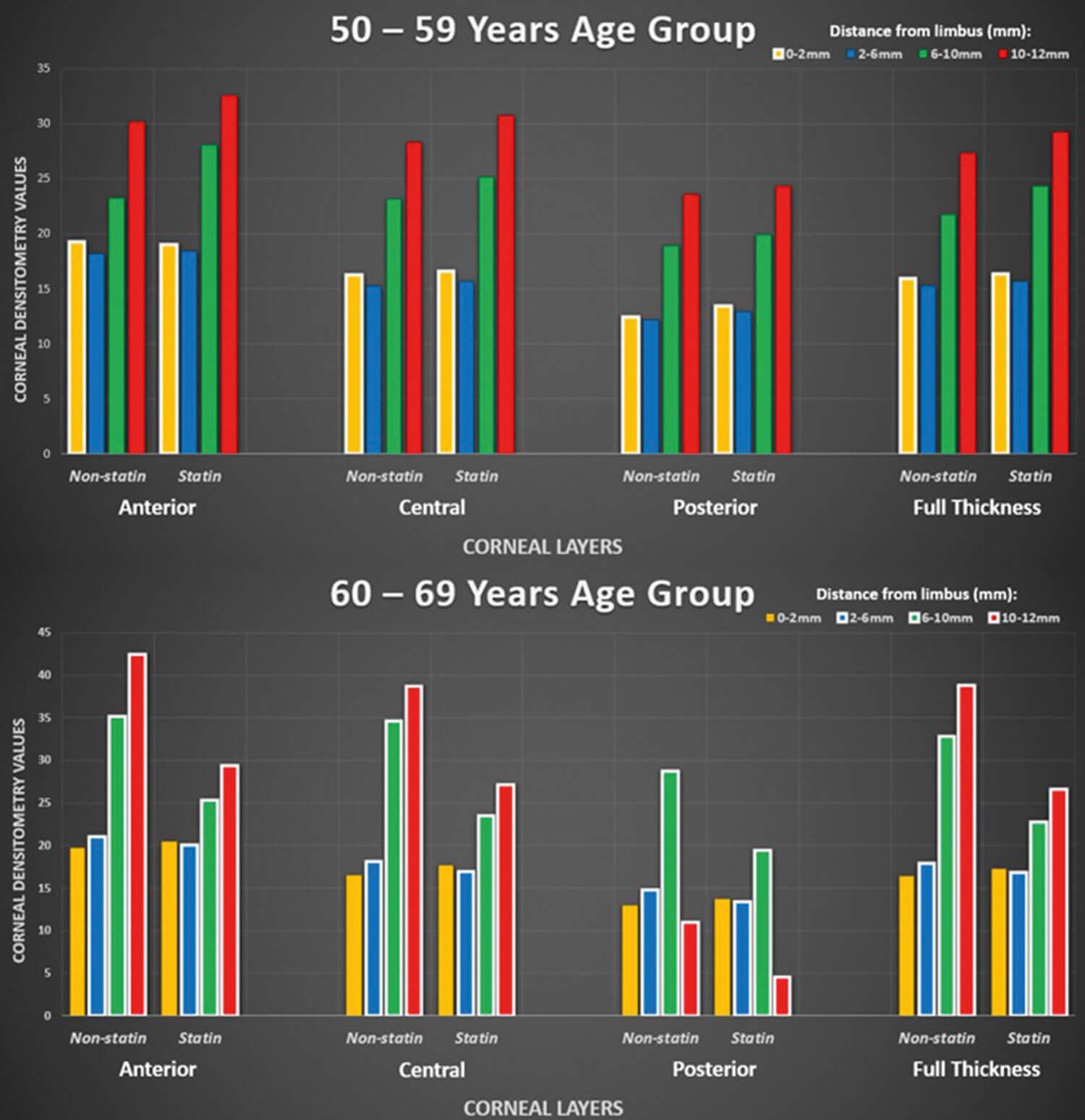
Figure 3: Graphs comparing densitometry values between 50–59 years and 60–69 years age groups.
A highly different trend was seen in the 60–69 years age group than the previous age group. Besides four results out of 32, all other values depicted lower densitometry levels for those in that age group that took statins. Furthermore, for the 6–10mm and 10–12mm concentric corneal zones, there was a statistically significant difference in densitometry between those taking statins and non-users across all levels of corneal depth, with the latter concentric zone showing higher levels of opacity (Figure 3). For the 6–10mm group, statin users expressed densitometry values of 22.78 compared to 32.86 for non-statin users. Similarly, for the 10–12mm group, statin users had values of 26.64 and 38.79 comparing statin users to non-users.
The results regarding the 70–79 years age group follow a similar pattern to those from the 60–69 years age group. This can be seen from the fact that, besides four results out of 32, all statin users in this age group had lower overall densitometry levels. However, none of these results were found to be statistically significant with full-depth p-values of concentric zones from 0–2mm to 10–12mm of 0.400, 0.222, 0.315, and 0.408. Finally, the results of the 80–89 years age group show that, similar to the previous two age groups, statin users overall had lower densitometry levels than non-users from the same group. Statistically significant results were seen for the 2–6mm (p=0.002) and 6–10mm (p=0.001) zones. Statin users for the former depicted densitometry values of 20.00 compared to 24.95 for non-users and the latter giving values of 33.49 for users compared to 47.25 for non-users.
This trend of reduced densitometry levels for statin users versus non-users in the 80–89 years age group persisted, with the exception of three results out of 32 obtained for all respective corneal regions. Specifically, the data from all 2–6mm and 6–10mm concentric corneal zones across all levels of depth were found to be statistically significant besides the posterior cornea 2–6mm zone (Figure 4).
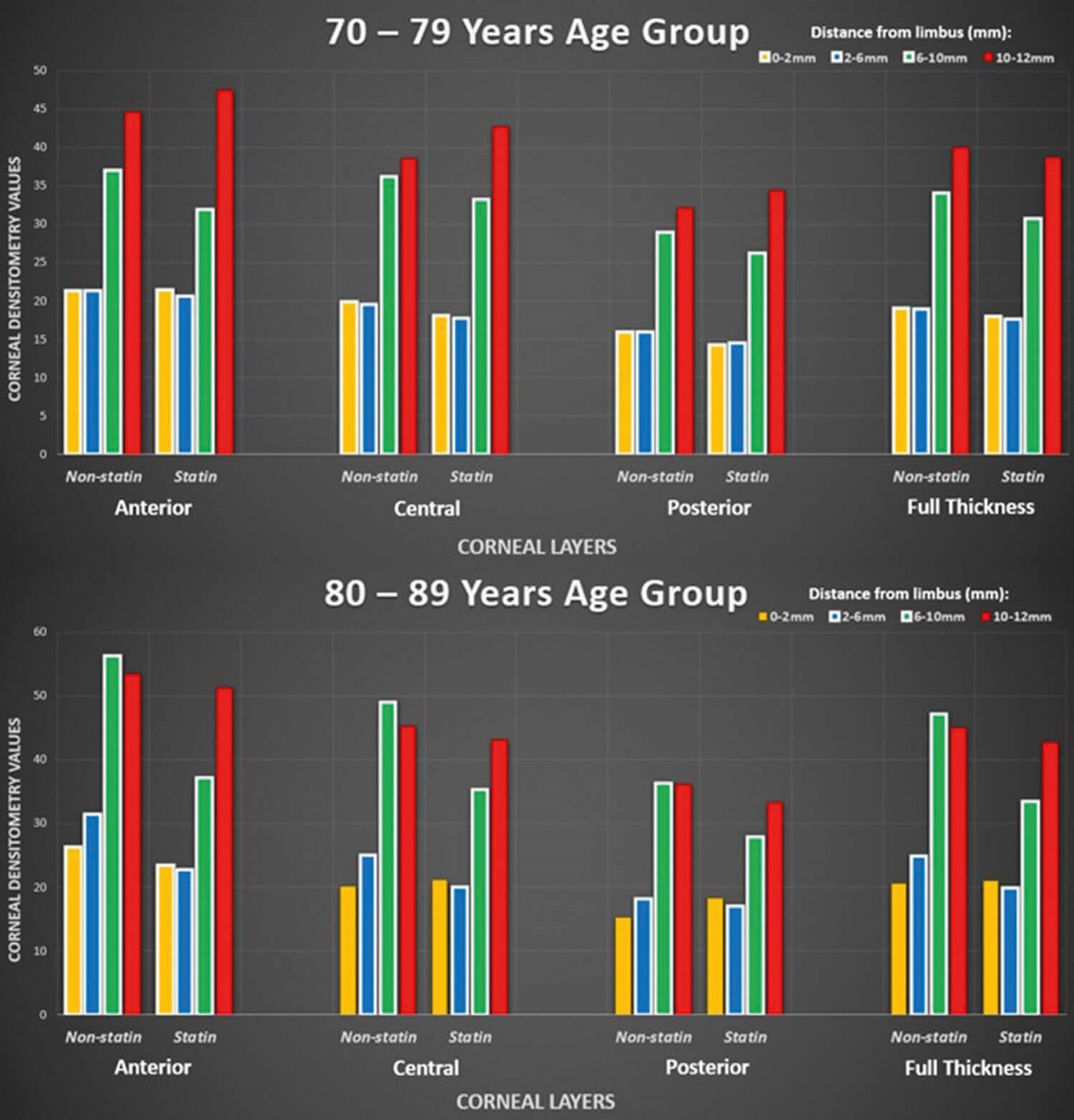
Figure 4: Graphs comparing densitometry values between 70–79 years and 80–89 years age groups.
To briefly summarise, in terms of the corneal densitometry results from the statin users compared to non-users, from the 60–69 years age group and above, the statin users were found to have lower densitometry levels than the respective non-users. Interestingly, the results for the 50–59 years age group indicate the opposite trend to that seen in the older age groups. The data from the 50–59 years age group suggested that there was an increase in corneal densitometry for statin users compared to non-users. All statistically significant results supported the hypothesis and were found in the 60–69 years and 80–89 years age groups, encompassing all layers of corneal depth. Of these trends, none of them were seen in the 0–2mm concentric zone but all other zones remain implicated.
Discussion
The results demonstrated above may be explained through previous studies undertaken to investigate the impact of statins on corneal clarity. Satpathy, et al. have highlighted the role of thrombin in corneal endothelial cell dysfunction via the destruction of tight junctions between cells. They demonstrated that statins may be linked to the halting of the proliferation of thrombin within the cornea, thereby maintaining the integrity of the endothelial cells [7]. In addition, Shivanna, et al. have shown that these medications, albeit in bovine samples in this case, may be implicated in preventing thrombin-induced loss of barrier integrity in corneal endothelial cells [8].
This area of research is of vital importance as it provides an insight into varying methods of treating corneal opacity. Certain corneal disorders which may contribute to corneal opacity are untreatable at the moment, so this study explores the possibility of utilising an alternative therapy in order to treat the loss of corneal clarity. Such diseases may include herpes simplex keratitis, an infective condition which leads to diffuse corneal scarring [10]. However, such a leap cannot be made as of yet due to the fact that this study has focused solely on corneas free from pathology. Further research into the application of statins in the context of various corneal illnesses may help to elucidate how beneficial this may be as a first or second-line option. This also depends on patient age as well as the regional spread of each condition across the cornea as this study has shown variable densitometry patterns throughout each respective corneal zone from the 0–2mm through to 10–12mm from the corneoscleral limbus across all age groups.
In addition to examining the impact of statin use as an environmental factor affecting the clarity of the cornea, systemic illness with an ocular component may play a role as well. For example, diabetes mellitus exhibits multisystem complications which already affect regions of the eye, such as the retina due to diabetic retinopathy. This may suggest that this illness is worth investigating in terms of the effects on corneal clarity. All diabetic patients are often prescribed statins upon diagnosis so the results from this study may have been influenced by the diabetic participants among the statin users within the cohort. Certain studies have already highlighted how conditions such as diabetic keratopathy may lead to reduced levels of corneal clarity based on damage affecting each corneal layer [11]. In addition, there has been evidence to suggest that other corneal conditions which may have a role including corneal ectasia following LASIK eye surgery as well as keratoconus. These conditions primarily affect the anterior cornea and alter the bulging of the cornea which ultimately increases corneal opacification levels, thus reducing overall clarity [12,13].
There were limitations associated with this investigation which were primarily due to practicality and relevant ethical approvals. For example, there was a large cohort of participants who were excluded from the study due to being in the wrong age group (below 20 or above 90) or due to existing corneal pathology. Although some participants may not be considered as having had corneal illness, they may still have had significant corneal scarring impacting corneal clarity, thereby skewing the densitometry analysis.
Conclusion
The investigation into the varying corneal densitometry values found for statin users compared to non-users revealed the following trends. Generally, based on the statistically significant results, the use of statins suggests increased corneal clarity across most concentric corneal zones (except the 0–2mm one) across all layers. This trend complies with the proposed hypothesis that statin use leads to increased corneal clarity across the age groups in this cohort. Although the hypothesis is supported, further research is required to determine the extent to which the results produced truly depict the reality seen in the UK population.
References
1. Forrester J, Dick A, McMenamin P, et al. The Eye: Basic Sciences in Practice (4th ed). Edinburgh, UK; Elsevier Ltd; 2016.
2. Massoudi D, Malecaze E, Galiacy S. Collagens and proteoglycans of the cornea: importance in transparency and visual disorders. Cell Tissue Res 2016;363(2):337–49.
3. Hassell J, Birk D. The Molecular Basis of Corneal Transparency. Exp Eye Res 2010;91(3):326–35.
4. Sivak J, Fini M. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res 2002;21(1):1–14.
5. Gong H, Wang Y, Qi X, et al. Differential response of lens crystallins and corneal crystallins in degenerative corneas. Exp Eye Res 2012;96(1):55–64.
6. Li S, Kim E, Bonanno J. Fluid transport by the cornea endothelium is dependent on buffering lactic acid efflux. Am J Physiol Cell Physiol 2016;311(1):116–26.
7. Satpathy M, Gallagher P, Jin Y, et al. Extracellular ATP opposes thrombin-induced myosin light chain phosphorylation and loss of barrier integrity in corneal endothelial cells. Exp Eye Res 2005;81:183–92.
8. Shivanna M, Jalimarada S, Srinivas S. Lovastatin Inhibits the Thrombin-Induced Loss of Barrier Integrity in Bovine Corneal Endothelium. J Ocul Pharmacol Ther 2010;26(1):1–10.
9. Oliveira C, Ribeiro C, Franco S. Corneal imaging with slit-scanning and Scheimpflug imaging techniques. Clin Exp Optom 2011;94(1):33–42.
10. Azher T, Yin X, Tajfirouz D, et al. Herpes simplex keratitis: challenges in diagnosis and clinical management. Clin Ophthalmol 2017;11:185–91.
11. Sayin N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World J Diabetes 2015;6(1):92–108.
12. Silverman R, Urs R, RoyChoudhury A, et al. Epithelial remodeling as basis for machine-based identification of keratoconus identifying keratoconus based on epithelial remodeling. Invest Ophthalmol Vis Sci 2014;55:1580–7.
13. Rocha KM, Perez-Straziota CE, Stulting RD, Randleman JB. SD-OCT analysis of regional epithelial thickness profiles in keratoconus, postoperative corneal ectasia, and normal eyes. J Refract Surg 2013;29:173–9.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME