The authors report on a study to examine the effects of the Mydriasert insert on time, effects, patient comfort and tolerability at Manchester Royal Eye Hospital.
Mydriasert is an insoluble ophthalmic insert indicated for mydriasis prior to ophthalmic surgery, which gradually releases its active ingredients of tropicamide 0.25mg and phenylephrine 5.4mg. The insert can only be used on suitable adult patients and must be removed within two hours of insertion. It is a one-step application; the suitably trained nurse places the insert in the lower fornix of the eyelid which remains until removal by the surgeon prior to surgery or by the nurse if the insert has been in situ for nearly two hours. If a patient is diagnosed as having ‘dry eye’ a drop of Sodium Chloride is instilled into the eye prior to the instillation of the insert.
Many studies have identified several factors that ought to be considered when using Mydriasert insert such as its effect in terms of pupillary dilation, its tolerability, reduction of additional equipment such as iris hook, and above all its cost [1-7]. Caruba et al. (2006) found that using the Mydriasert insert required less nurse gestures per patient than the drop protocol [2]. The study by Shah et al. (2015) aimed to evaluate the cost of Mydriasert compared with conventional mydriatic eye drops to induce pupil dilation prior to cataract surgery [3]. They demonstrated the annual total costs decreased by 18% and annual total nurse time decreased from 235.1 hours to 44.1 hours over one year (2012–2013) when Mydriasert substituted mydriatic eye drops.
“Several factors that ought to be considered when using Mydriasert insert such as its effect in terms of pupillary dilation, its tolerability, reduction of additional equipment such as iris hook, and above all its cost.”
Aim
This study aimed to examine the effects of the Mydriasert insert on time, effectiveness, patient comfort and tolerability at Manchester Royal Eye Hospital. In selected appropriate patients Mydriasert insert was used instead of the preservative free cyclopentolate and phenylephrine minims for the effect of mydriases pre-cataract surgery.
Method
The introduction of Mydriasert insert was piloted and audited at the Private Patient Centre, Manchester Royal Eye Hospital. The population consisted of n=50 patients who were admitted for cataract surgery. As this was a new drug to the Trust, the Medicines Management Committee was consulted, and permission was granted to purchase the medication and commence its introduction. An algorithm was produced by the pharmacy team of the Medicines Management Committee which detailed the inclusion and exclusion criteria for the nursing team.
A protocol was devised, and training given for three ophthalmic trained nursing staff to be able to introduce and remove the Mydriasert insert from the lower fornix of the eyelid. Nurses were also given information on the pharmacological aspects and limitations of the drug. Two participant ophthalmic consultants were advised on how to prescribe the insert and given information regarding the inclusion and exclusion criteria. The pharmacy algorithm was referred to on choosing appropriate patients and strict documentation was indicated regarding times of instillation and removal.
Data collection
Data was gathered using a questionnaire, Trust medication drug chart and a timing slip placed on each set of notes detailing insertion time, removal time ‘due’, actual removal time and place of removal. The nurse was asked to measure pupil size pre and post insertion to measure efficacy of the insert using the pentorch scale (Glasgow Coma Scale 1974). The questionnaire was completed by the three trained nurses who had received the training through one on one interview with the patients during the admission process and again following the insertion of the insert.
Ethical considerations
All patient participants were informed of the purpose of the introduction of the drug insert and the study was based on voluntary participation. The Trust gave permission for this work by way of the Medicines Management Committee.
Results
The study population included a total of n=50 patients, 86% female and 14% male with an average age of 59 years. Of the sample group, 10% were diagnosed with dry eye and so had a drop of Sodium Chloride instilled into the lower fornix prior to the insertion of Mydriasert. Ninety percent of inserts were removed by the surgeon pre-administration of local or general anaesthetic; 6% were removed by appropriately trained nursing staff on the ward due to time constraints (Mydriasert had been in situ for nearly the two-hour deadline) and 4% fell out of the patient’s eye preoperatively. On average, the Mydriasert insert was in situ for approximately 73 minutes before removal, well within the deadline for removal. To create a baseline, the average size of pupil was measured before insertion of Mydriasert (Figure 1).
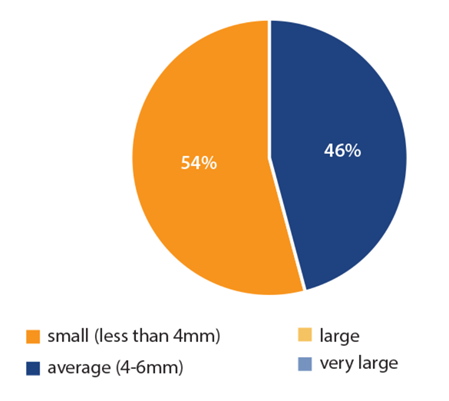
Figure 1: Size of pupil pre-insertion of Mydriasert.
The average size of pupil was small (less than 4mm) or average (4-6mm).
No participants had a large pupil.
In comparison, following the insertion of Mydriasert and at a minimum of 45 minutes following the insertion (pupil check point), it was noted that 84% of patients had very large pupils (Figure 2).
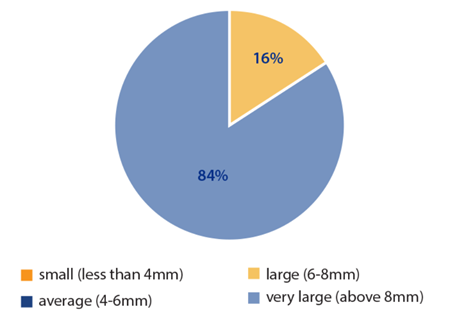
Figure 2: Average size of pupil after 45mins of insert in situ; 16% of the cohort
had a large pupil and 84% were classed as having a very large pupil.
We also measured the overall ocular tolerance as described by the patient at the end of the observation period (45 minutes). This was measured on a scale of 1-5 where 1 is poor tolerance and 5 is excellent tolerance to the insert (Figure 3).
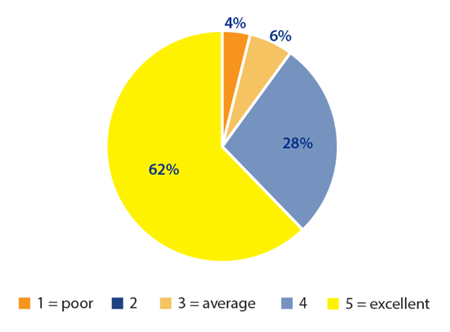
Figure 3: Overall patient ocular tolerance of the insert.
Specifically, we looked at three areas of discomfort the patient may experience while the insert is in situ. These areas were stinging sensation (Figure 4), watering of eyes (Figure 5) and foreign body sensation (Figure 6).
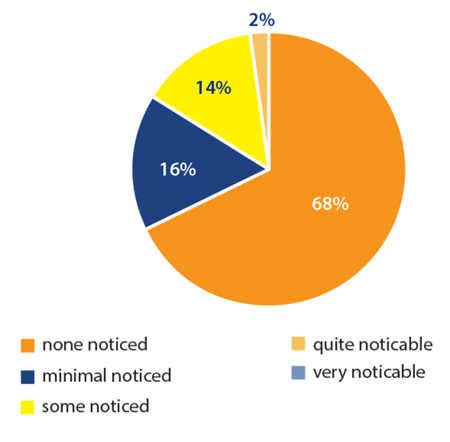
Figure 4: Patient ocular tolerance of the insert in relation to stinging sensation of the eye.
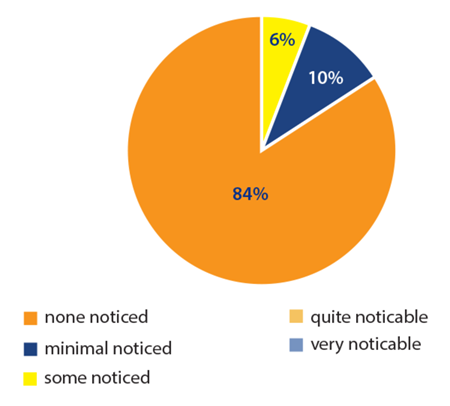
Figure 5: Patient ocular tolerance of the insert in relation to watering sensation of the eye.
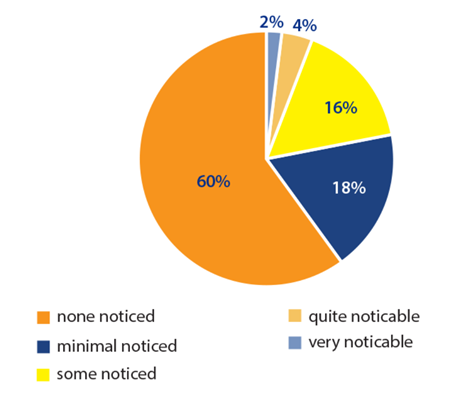
Figure 6: Patient ocular tolerance of the insert in relation to foreign body sensation of the eye.
We also noted the ease of insertion of Mydriasert from the patient and practitioner perspective (Figures 7 and 8).
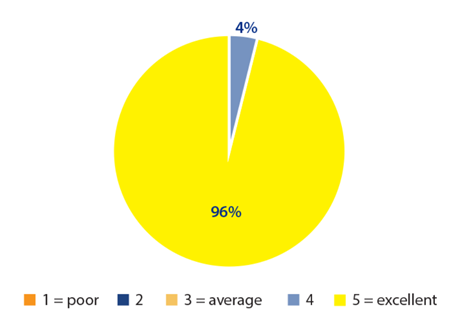
Figure 7: Patient comfort level when Mydriasert inserted.
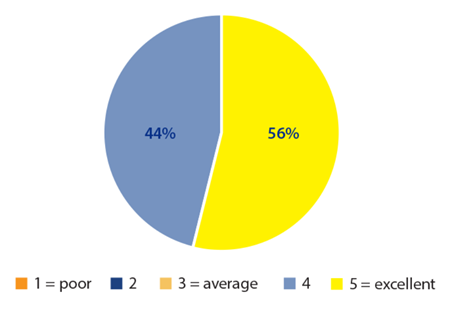
Figure 8: Ease of insertion of Mydriasert into the lower fornix by the team.
Discussion
Our findings indicated that the Mydriasert insert achieves good pupil dilatation before cataract surgery, comparable to the standard set of preoperative mydriatic agents.
Our investigation supports previous studies which demonstrated the efficacy of the Mydriasert insert. Indeed, in our study, using a single insert of the drug it was found the pupil size was large and 84% were classed as having a very large pupil. These are consistent with the findings of Morgado et al. (2010) [4]. In their comparative study of drops vs. Mydriasert they found that pupillary dilation and a stable pupil was best in the Mydriasert group (n=90). This was supported by Levet et al. (2004), who also found that maximal mydriasis was significantly greater in the Mydriasert group regardless of the frequency of instillation in the drop group [5].
However, in the present study we examined the effects of the Mydriasert insert and tolerability. We found that the majority patients (n= 62%) tolerated the drug well in terms of foreign body sensation, watering and stinging of the eyes.
The NHS undertakes an enormous amount of cataract removals per year, more than any other surgical procedure – 395,661 in 2015-16 (RNIB 2016). The number of operations has been increasing in recent years due to the ageing population. However, financial pressures the NHS faces mean the rationing and postponement of second eye cataract surgery [8]. In turn, cost saving methods that could progress the efficiency of the surgery should be considered. Dilation of the pupil is imperative preoperatively prior to cataract surgery to aid ease of lens removal [9,10] and reduction in the need for iris hooks [3]. Our study demonstrated no use of iris hook. This suggests that Mydriasert insert may reduce the frequency of iris hook, and subsequently reduce the associated cost by reducing the surgeon’s time.
Currently, within our Trust, the method for achieving mydriasis is the use of two dilating minims drops of Cyclopentolate 1% and Phenylephrine 2.5% instilled on three separate occasions in 15-30 minute intervals. Sometimes these drops are not re-administered in a timely manner and the size of the pupil may have been reduced, causing the recognisable complication of posterior capsule rupture [11]. In our study, it took an average of 45 minutes to achieve optimal dilation of the pupil. Caruba et al. (2006) found that using the Mydriasert insert required less nurse gestures per patient than the drop protocol [2]. Therefore, it should consider that although the Mydriasert drug is more expensive than existing treatments, overall cost is reduced due to increasing nursing efficiency and minimising other workload, as well as potential reduction in surgical time and possible complications, which is a key cost driver. In summary, the effects of the Mydriasert insert are comparable to those of standard mydriatic eyedrops in terms of safety and pupil dilation, with no safety concerns and minimal patient intolerance. Therefore, we believe that Mydriasert is a good alternative option for pupillary dilation prior to cataract surgery.
Conclusion and recommendations
In conclusion, Mydriasert produces effective and pupillary dilation. Side-effects are limited and ease of use by staff is highlighted. Although the data does not measure time saved by nursing staff it can be noted that the insertion of the Mydriasert was a one-step procedure as opposed to the multiple instillation of eyedrops on several occasions (as is current practice) which improves efficiency and could allow nurses to spend more time on other activities. Limitations of this paper include that it is not a direct comparative study comparing a Mydriasert group with a parallel eyedrop control group. This work would allow further study to take place in comparing nursing time between Mydriasert insert versus mydriatic drops that are traditionally used to study the cost-effectiveness of Mydriasert.
References
1. Cagini C, Sbordone GB, Ricci AL, Menduno P. Efficacy and safety of limbal anaesthesia for clear cornea phacoemulsification. Acta Ophthalmol Scand 2014;82(3Pt1):315-6.
2. Caruba T, Couffon-Partant C, Oliary J, et al. Efficacy and efficiency of preoperative mydriasis: drops versus ocular insert. J Fr Ophtalmol 2006;29(7):789-95.
3. Shah A, Sukhvinder J, Lee N. Mydriasert pupillary dilation for cataract surgery: an economic and clinical study. BMC Ophthalmol 2015;15:56.
4. Morgado G, Barros P, Martins J, et al. Comparative study of mydriasis in cataract surgery: topical versus Mydriasert versus intracameral mydriasis in cataract surgery. Eur J Ophthalmol 2010;20(6):989-93.
5. Levet L, Touzeau O, Scheer S, et al. Etude de la dilatation pupillaire par I’insert ophtalmique Mydriasert. J Fr Ophtalmol 2004;27(10):1099-108.
6. Saenz-de-Viteri M, Fernadez-Robredo P, de Nova E, et al. Comparative study measuring the dilatory effect of a mydriatic device (Mydriasert) versus topical drops. Int J Ophthalmol 2013;6(6):801-4.
7. Torron C, Calvo P, Ruiz-Moreno O, et al. Use of a new ocular insert vs convential mydriasis in cataract surgery. Biomed Research International 2016;2013:849349.
8. Royal College of Ophthalmologists. Cataract surgery must be determined on clinical need and not rationed due to funding restrictions.
www.rcophth.ac.uk/2016/08/
cataract-surgery-must-be-determined-
on-clinical-need-and-not-rationed-
due-to-funding-restrictions/
9. Royal College of Ophthalmologists. Cataract Surgery Guidelines 2010
www.rcophth.ac.uk/wp-content/
uploads/2014/12/2010-SCI-069-Cataract-
Surgery-Guidelines-2010-SEPTEMBER-2010-1.pdf
10. National Institute for Health and Care Excellence. Cataracts in adults: management 2017
www.nice.org.uk/guidance/ng77/
resources/cataracts-in-adults-
management-pdf-1837639266757
11. Narendran N, Jaycock P, Johnston RL, et al. The Cataract National Dataset electronic multicentre audit of 55567 operations: risk stratification for posterior capsule rupture and vitreous loss. Eye (Lond) 2009;23(1):31-7.
(All links last accessed May 2018)
Acknowledgment:
The authors would like to thank the nurses and surgeons from Manchester Royal Eye Hospital who took part in this study for their devoted work and participation, and particularly Ali Yagan for advising on this article.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME







