Traditionally, a newly diagnosed glaucoma patient would be treated first with medical therapy. As the disease progressed or the initial intervention failed to adequately control intraocular pressure (IOP), clinicians would add more drops, selective laser trabeculoplasty (SLT), repeated SLT and still more drops, with surgery as a last resort.
This treatment pathway made sense because the risks of side-effects and potential harm from trabeculectomy surgery were significant. But the risk-benefit ratio, which defines all of our decisions in medicine, is shifting rapidly.
Following the results of the Laser in Glaucoma and Ocular Hypertension (LiGHT) Trial, which found that there was a 97% probability of primary SLT being more cost-effective than eye drops [1], the UK National Institute of Clinical Excellence (NICE) mandated that glaucoma patients should be offered laser as primary therapy. Longer follow-up in the LiGHT Trial has continued to validate the benefits of primary laser treatment, which reduces the need for incisional glaucoma surgery and cataract surgery over six years [2].
We have also seen the introduction of minimally invasive glaucoma surgery (MIGS) procedures. While these procedures tend to provide smaller marginal gains than conventional trabeculectomy, they are easier to perform and lower risk. MIGS has encouraged a shift towards more combined surgery and even standalone MIGS performed earlier in the course of the disease, enabling us to consider surgery for potentially less benefit.
In fact, we now have good evidence that early, non-medical IOP control offers better long-term disease control and preservation of vision – which is what matters most to patients. For example, we know from the LiGHT Trial that patients had better visual field preservation with laser than with drops even when both groups had similar IOP [2]. We also saw better visual field preservation in the HORIZON trial among patients undergoing phacoemulsification with a Hydrus microstent (Ivantis) versus phaco alone [3]. In addition to maintaining their vision and independence, patients also highly value being free from eye drops [4], so earlier surgical intervention that postpones or reduces the need for drops has the potential to improve the patient experience.
Nevertheless, the requirement for some MIGS procedures to be performed with cataract surgery, the incisional nature of operating theatre-based procedures, surgical trauma and variable healing responses are all limitations of MIGS.
Current and near-future paradigms
My current paradigm for a newly diagnosed glaucoma patient is typically SLT, repeat SLT, then a once-daily preservative-free (PF) prostaglandin. If the pressure increases or is still not well controlled after that, I add a PF low-dose, slow-release beta blocker once daily. The next step varies, depending on the patient and their disease stage. At any stage in this paradigm, if the patient needs cataract surgery, I’ll offer a combined procedure with MIGS.
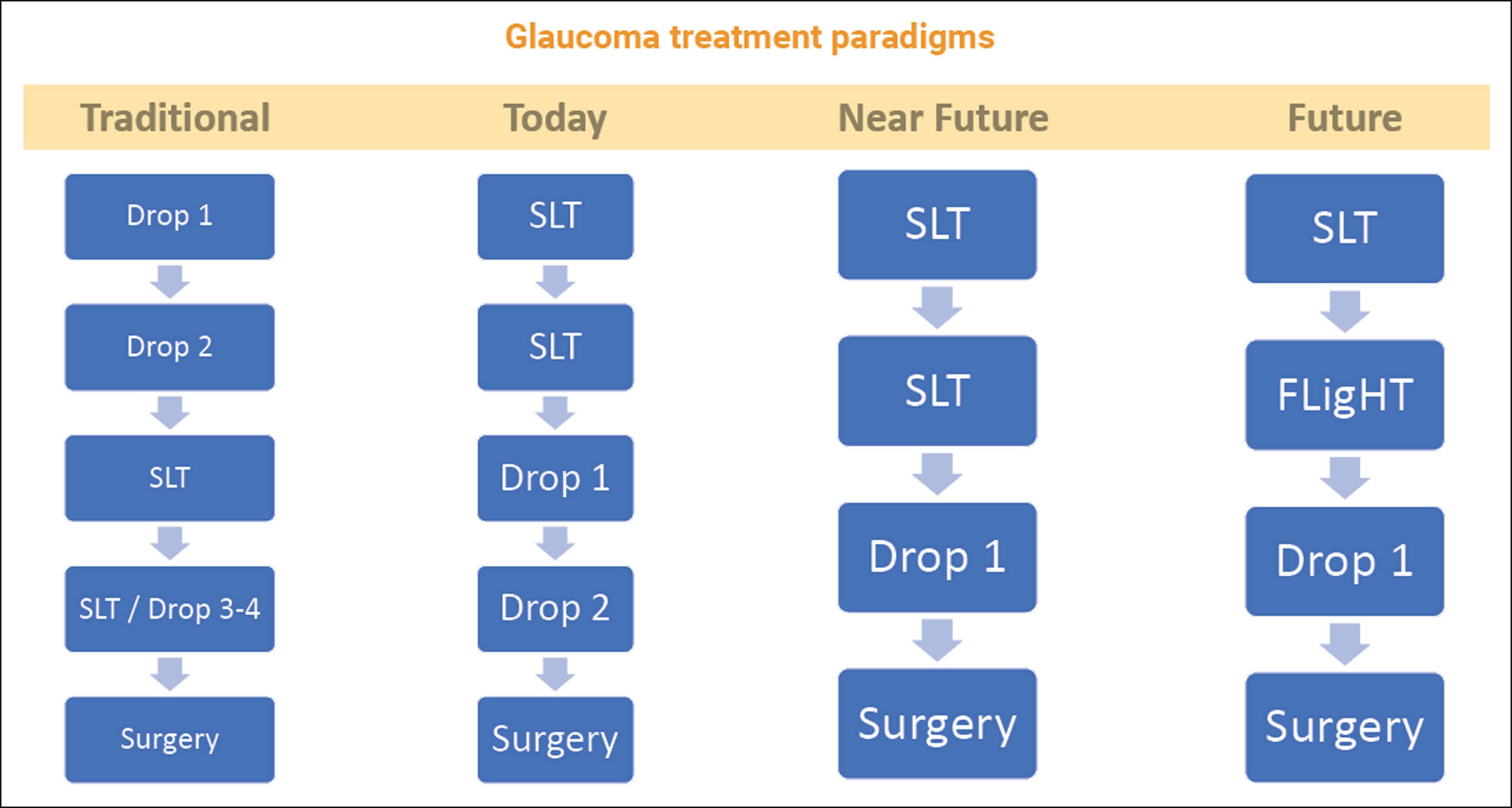
Figure 1: The treatment paradigm in glaucoma is shifting rapidly towards earlier intervention and non-invasive treatments.
My preference for earlier surgical intervention will only continue to accelerate over the near and longer term (Figure 1) with new surgical options on the horizon such as the MINIject (iStar Medical) and excimer laser trabeculostomy (ELT). While both of these ab-interno procedures carry the risks of incisional surgery, they are less invasive than conventional trabeculectomy and have shown good efficacy in initial studies [5,6].
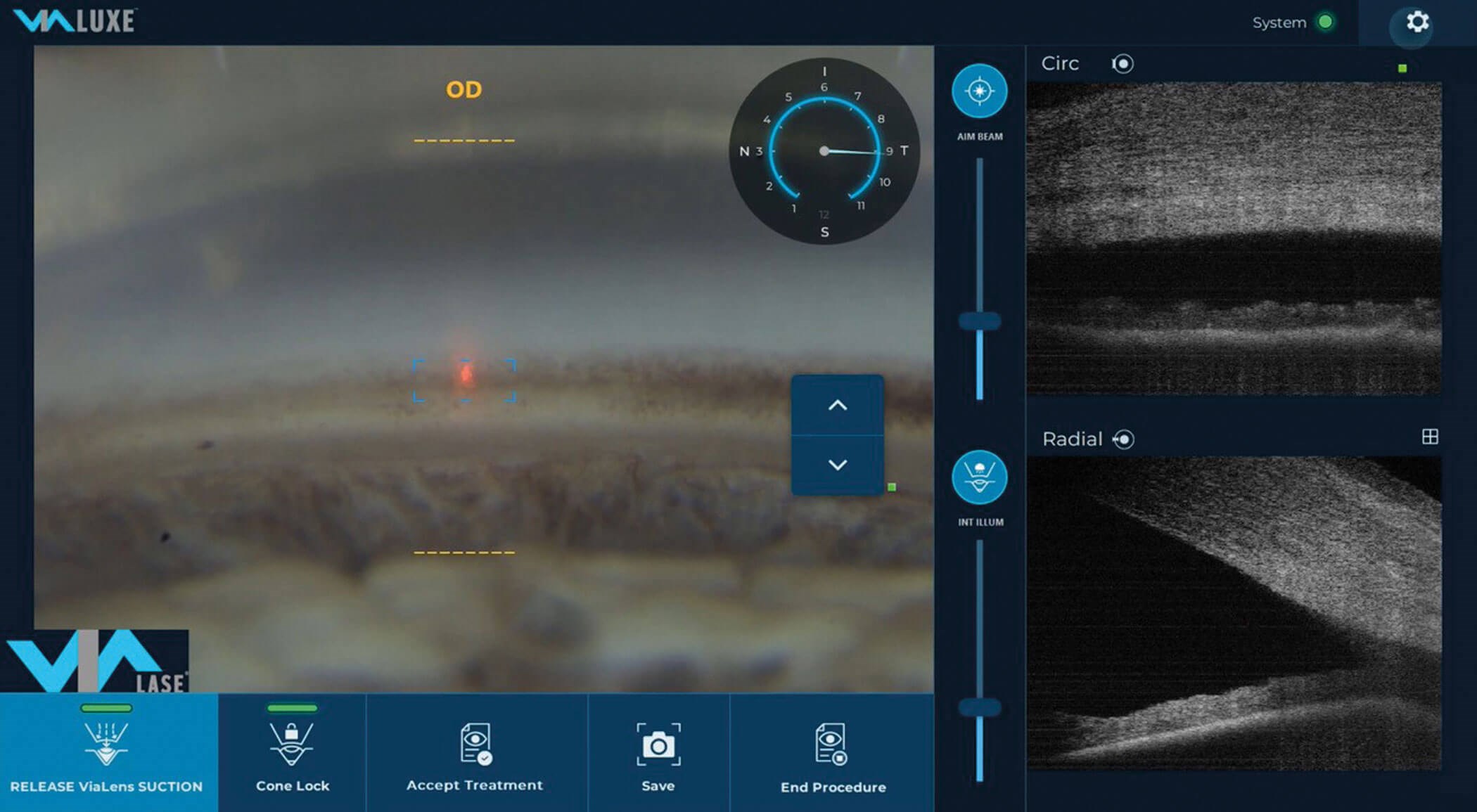
Figure 2: The FLigHT treatment (ViaLase) non-invasively creates
precise trabeculotomy channels in the trabecular meshwork.
I’m also very enthusiastic about a new laser treatment, femtosecond laser image-guided, high-precision trabeculotomy (FLigHT), that has the potential to deliver surgery-like results at much lower risk. FLigHT is performed with the ViaLuxe laser system (ViaLase). This laser treatment accomplishes what is essentially an ab-externo surgical procedure – creating trabecular meshwork (TM) drainage channels – but without opening the eye (Figure 2). One of the great advantages of a femtosecond laser is that the laser beam can pass through tissue without cutting it to reach a precisely targeted location. While these capabilities are now taken for granted in the fields of cataract and refractive surgery, they are novel in glaucoma.
With the safety inherent in a non-incisional procedure, I believe FLigHT could potentially become a first-line treatment and will easily become a second-line treatment after SLT in the near future. Two-year data from the first studies in humans showed that 82% of eyes achieved a >20% reduction and 53% of eyes achieved a >25% reduction in IOP [7]. The combination of femtosecond laser precision and micron-accurate image guidance in the same platform is novel, and the visualisation excellent.
For now, SLT will likely continue to have a primary position in our treatment paradigm because it is inexpensive, easy and safe. But we know that SLT doesn’t work for everyone and it doesn’t work forever, so there remains a need for new non-invasive treatments for glaucoma. The introduction of femtosecond lasers in glaucoma could be the biggest paradigm shift in how we treat early to moderate glaucoma that we’ve seen in a generation.
Considering cost
The shift toward a more interventional mindset in glaucoma has occurred somewhat unevenly around the world, partly due to health system factors such as reimbursement and financial incentives. In the UK, where 90% of glaucoma care is provided through the NHS and large hospitals have largely been willing to absorb the cost of new technology, MIGS procedures have been widely available to our patients. In contrast, MIGS procedures have been almost completely unavailable under some European healthcare systems and available only to those with private insurance in others.
When we think about the cost of new treatments that rely on new laser technology, it will be important to consider how much of the price tag is for consumables and how much gets amortised over many procedures. In countries like the UK, a large capital expenditure can be relatively easily amortised over many patients, but a high per-patient consumable cost may be untenable. And of course, we have to consider the cost-effectiveness. If a future glaucoma treatment could be truly ‘one-and-done’, that would be a huge improvement even over less expensive but chronic or repeated treatments. My expectation is that the cost of innovative new treatments will not be as much of a barrier to early intervention as some might expect.
Conclusion
We have increasingly robust evidence that early IOP control provides better disease control. And prolonged disease control ensures that we can avoid more invasive, riskier surgical measures that have the potential to cause harm even as they control pressure. Although primary surgical control is not something I see for the near future, a non-incisional, non-invasive treatment with surgical-like results could completely transform when and how we intervene. We have already travelled some way down this path with MIGS, and I believe other technologies yet to come will take us even further down the path of early intervention.
References
1. Gazzard G, Konstantakopoulou E, Garway-Heather D, et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): A multicentre randomized controlled trial. Lancet 2019;393:1505–16.
2. Gazzard G, Konstantakopoulou E, Garway-Heather D, et al. Laser in Glaucoma and Ocular Hypertension (LiGHT) trial: Six-year results of primary selective laser trabeculoplasty versus eye drops for the treatment of glaucoma and ocular hypertension. Ophthalmology 2023;130(2):139–51.
3. Montesano G, Ometto G, Ahmed IIK, et al. Five-year visual field outcomes of the HORIZON trial. Am J Ophthalmol 2023;251:143–55.
4. Safitri A, Konstantakopoulou E, Hu K, Gazzard G. Treatment expectations in glaucoma: What matters most to patients? Eye (Lond) 2023;24:1–9.
5. De Francesco T, Ahmed IIK. Surgical augmentation of the suprachoroidal space: A novel material and implant. Clin Ophthalmol 2023;17:2483–92.
6. Berlin MS, Shakibkhou J, Tilakaratna, et al. Eight-year follow-up of excimer laser trabeculostomy alone and combined with phacoemulsification in patients with open-angle glaucoma. J Cataract Refract Surg 2022;48(7):838–43.
7. Nagy ZZ, Kranitz K, Ahmed IK, et al. First-in-human safety study of femtosecond laser image guided trabeculotomy for glaucoma treatment: 24-month outcomes. Ophthalmol Sci 2023;3(4):100313.
Editorial support for this article was provided by contributing editor Jan Beiting.
Declaration of competing interests: In the last 10 years, Gus Gazzard has had, amongst others, the following commercial affiliations: Consultant /Advisor, Lecture Fees / Speakers Bureau (Alcon Laboratories / Ivantis); and Consultant / Advisor (ViaLase).
COMMENTS ARE WELCOME