Rod McNeil provides an update on a promising bispecific antibody recently approved for treatment of neovascular age-related macular degeneration (nAMD) and diabetic macular oedema (DMO) and considers emerging developments in biosimilars to established anti-vascular endothelial growth factor (anti-VEGF) therapies, including regulatory positions on biosimilar products.
Fast-track appraisal of faricimab for nAMD and DMO
The UK Medicines and Healthcare products Regulatory Agency (MHRA) approved intravitreal faricimab (Vabysmo, Roche) for treatment of adult patients with nAMD and visual impairment due to DMO in May 2022 [1]. It is the first treatment licensed by the MHRA through its participation in the Access Consortium, an international regulatory collaboration that aims to provide faster access to high-quality treatments. Faricimab was subsequently approved by the National Institute for Health and Care Excellence (NICE) as a first-line treatment option for adults with nAMD or DMO in late June 2022, under its fast-track appraisal process for technologies that offer exceptional value for money [2,3].
Faricimab is an intraocular bispecific antibody that acts through inhibition of two distinct pathways by neutralisation of both angiopoietin-2 (Ang-2) and vascular endothelial growth factor A (VEGF-A) [1]. Ang-2 causes vascular instability by promoting endothelial destabilisation, pericyte loss, and pathological angiogenesis, thus potentiating vascular leakage and inflammation [1]. By dual inhibition of Ang-2 and VEGF-A, faricimab reduces vascular permeability and inflammation, inhibits pathological angiogenesis and restores vascular stability [1].
The recommended dosing schedules for faricimab in treating nAMD and DMO are summarised in Figure 1 [1]. The product label states that faricimab must be administered by a qualified healthcare professional trained in intravitreal injections, reflecting UK clinical practice where most intravitreal injections are given by specialist nurses and optometrists [1].
Final guidance from NICE, published 29 June 2022, approved faricimab as a treatment option for adults with nAMD or DMO if it is used in the same population as aflibercept (Eylea, Bayer) and ranibizumab (Lucentis, Novartis) [2,3]. Evidence from clinical trials shows that faricimab is as effective as aflibercept in improving vision and reducing vision loss and had similar adverse events, while an indirect comparison of faricimab with ranibizumab also suggests similar clinical effectiveness.
In all four pivotal phase 3 trials (3220 patients enrolled across all four trials), patients treated with faricimab 6 mg up to every 16 weeks (Q16W) achieved noninferior vision gains compared with aflibercept 2 mg given every eight weeks (Q8W) at one year [4,5]. Efficacy and durability outcomes across the four studies are summarised in Tables 1 and 2 [1].
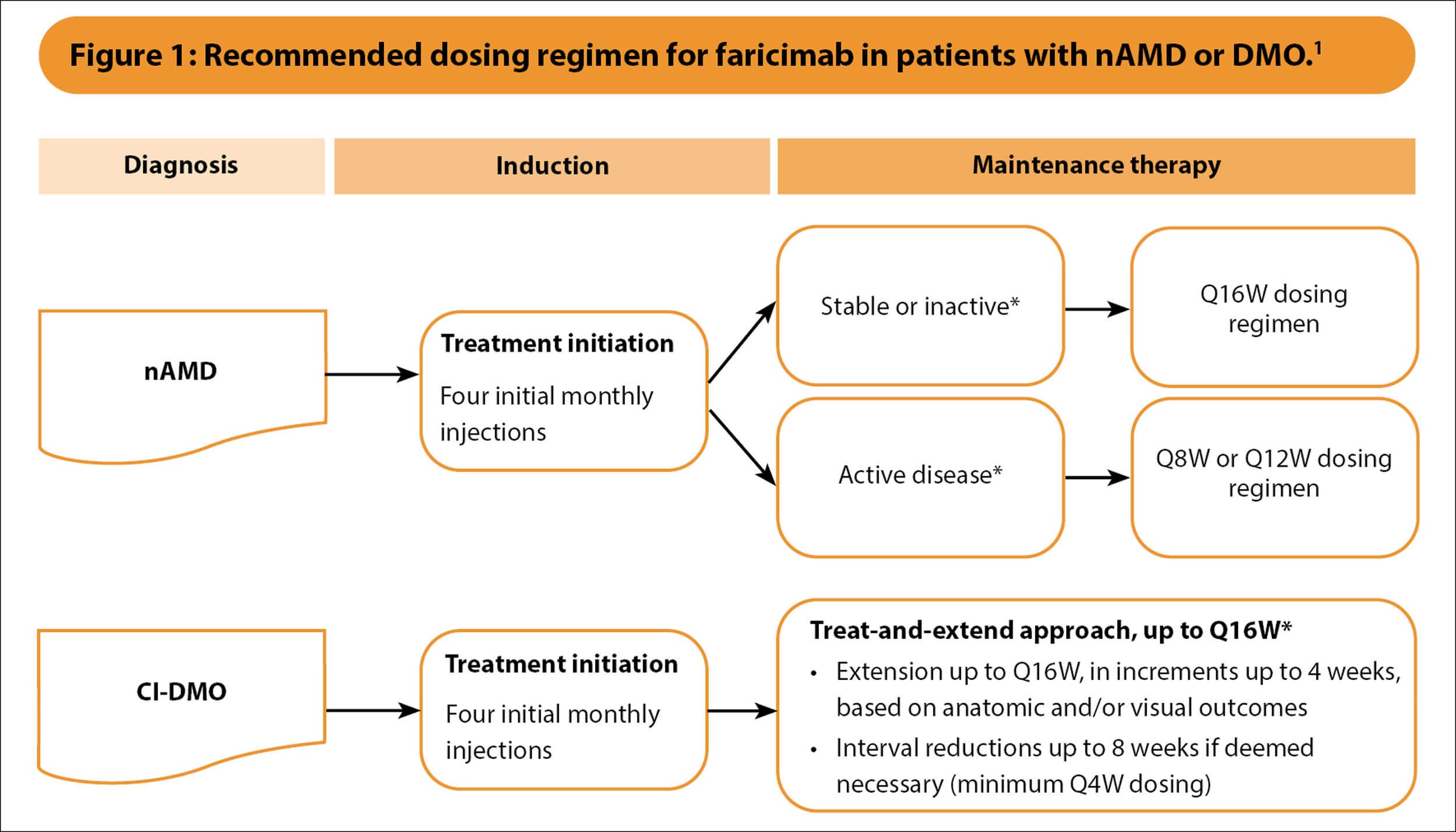
*Based on optical coherence tomography and visual acuity evaluations.
CI-DMO, centre-involving diabetic macular oedema; nAMD, neovascular age-related macular degeneration; Q4W, every 4 weeks; Q8W, every 8 weeks; Q12W, every 12 weeks; Q16W, every 16 weeks.
1 Vabysmo 120 mg/mL solution for injection [Summary of Product Characteristics]; Roche Products Limited, UK, May 2022.
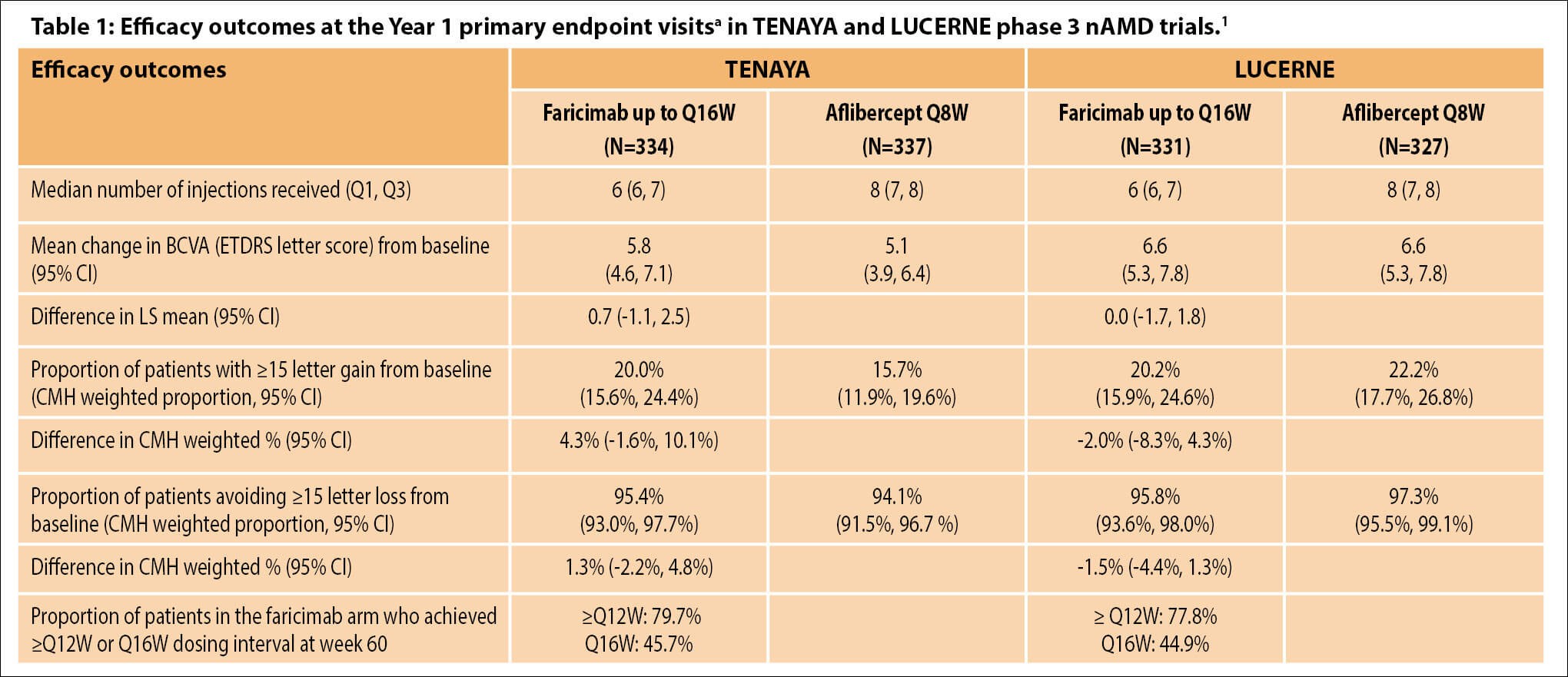
aAverage of weeks 40, 44 and 48.
BCVA, best-corrected visual acuity; CI, confidence interval; CMH, Cochran–Mantel–Haenszel method; ETDRS, Early Treatment Diabetic Retinopathy Study; LS, least square; nAMD, neovascular age-related macular degeneration; Q1, 1st quartile; Q3, 3rd quartile; Q8W, every 8 weeks; ≥Q12W, every 12 weeks or longer; Q16W, every 16 weeks.
1 Vabysmo 120 mg/mL solution for injection [Summary of Product Characteristics]; Roche Products Limited, UK, May 2022.
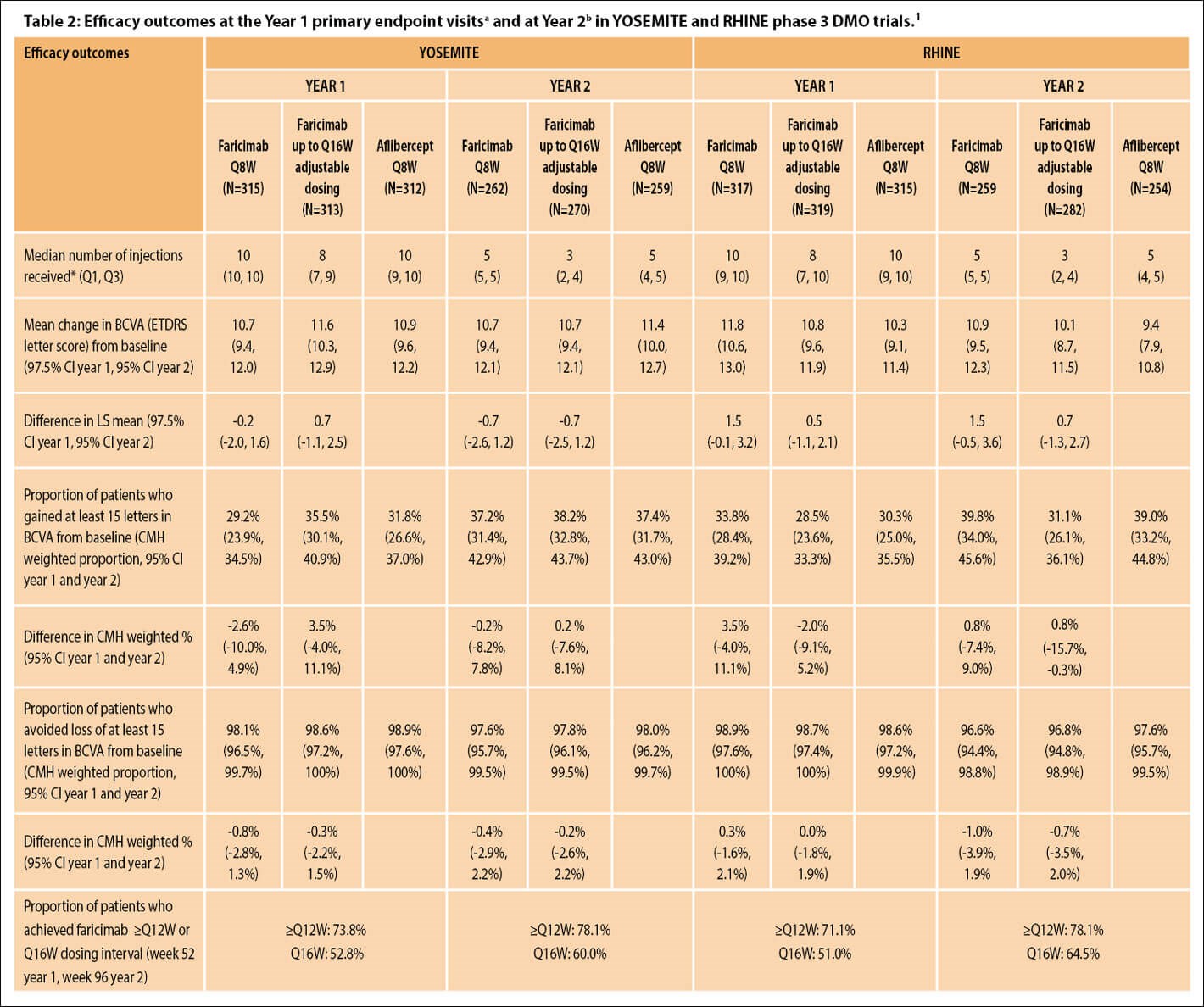
aAverage of weeks 48, 52 and 56, bAverage of weeks 92, 96 and 100.
*Median number of injections received for Year 1 corresponds to the period of baseline through Year 1, and for Year 2 corresponds to the period from Year 1 to Year 2
BCVA, best-corrected visual acuity; CI, confidence interval; CMH, Cochran–Mantel–Haenszel method; DMO, diabetic macular oedema; ETDRS, Early Treatment Diabetic Retinopathy Study; LS, least square; nAMD, neovascular age-related macular degeneration; Q1, 1st quartile; Q3, 3rd quartile; Q8W, every eight weeks; ≥Q12W, every 12 weeks or longer; Q16W, every 16 weeks.
1 Vabysmo 120 mg/mL solution for injection [Summary of Product Characteristics]; Roche Products Limited, UK, May 2022.
Faricimab is expected to be cost saving or have similar costs compared with aflibercept or ranibizumab and is likely to deliver similar health benefits [2,3]. It is recommended that if patients and their clinicians consider faricimab to be one of a range of suitable treatments (including aflibercept and ranibizumab), choose the least expensive treatment, taking account of administration costs, dose, price per dose and commercial arrangements [2,3]. Following a positive NICE recommendation, NHS England/commissioners have committed to providing funding for the technology within 30 days of final guidance publication.
The Committee for Medicinal Products for Human Use (CHMP) in July 2022 recommended EU approval of faricimab for the treatment of nAMD and DMO.
Pivotal clinicals trials evaluating faricimab in nAMD and DMO
• One-year results from phase 3 TENAYA and LUCERNE trials of faricimab for nAMD
TENAYA and LUCERNE investigated faricimab using an individualised treatment interval up to Q16W versus fixed aflibercept Q8W (after four and three initial monthly doses, respectively) in treatment-naïve patients with choroidal neovascularisation (CNV) secondary to AMD (nAMD) (best-corrected visual acuity [BCVA] of 24–78 Early Treatment Diabetic Retinopathy Study [ETDRS] letters) [4]. The primary endpoint was mean change in BCVA from baseline averaged over weeks 40, 44 and 48 (prespecified noninferiority margin of 4 letters).
Following initial loading doses and disease activity assessments at weeks 20 and 24, patients in the faricimab arm were treated with fixed Q16W, Q12W, or Q8W dosing intervals until week 60, with dosing determined by disease activity (central subfield thickness [CST] and visual acuity [VA] change). The study protocols did not permit rescue injections. From week 60, patients in the faricimab arm were treated according to a flexible dosing regimen between Q8W and Q16W up to week 108.
Robust visual gains and CST reductions with faricimab were comparable to aflibercept through week 48, with ~45% of faricimab patients on Q16W dosing and almost 80% on ≥Q12W dosing [4,6]. Faricimab was well tolerated, and no cases of retinal vasculitis or occlusive retinal vasculitis were reported.
Professor Ian A Pearce, Consultant Ophthalmologist, St Paul’s Eye Unit, Royal Liverpool University Hospital, commented: “The introduction of faricimab as a treatment option for nAMD is likely to have a significant positive impact on capacity and care pathway by reducing the frequency of injections and visits compared with current standard care treatment options.”
• Two-year results from phase 3 YOSEMITE and RHINE trials of faricimab for DMO
YOSEMITE and RHINE investigated faricimab treat-and-extend (T&E)–based dosing with up to Q16W intervals and fixed Q8W dosing versus aflibercept fixed Q8W in anti-VEGF treatment-naïve or previously treated (~22%) patients with centre-involving DMO (CST ≥325 µm and BCVA 25–73 ETDRS letters) [5,7]. Treatment was initiated with a loading regimen of four, six and five consecutive monthly injections in the faricimab T&E, faricimab Q8W and aflibercept Q8W arm, respectively. Treat-and-extend–based dosing regimen intervals were adjusted (from Q4W up to Q16W) based on CST and BCVA change at active dosing intervals.
Vision gains from baseline in both faricimab groups were noninferior to aflibercept Q8W at one year (averaged over weeks 48, 52 and 56) and were maintained through year two (averaged over weeks 92, 96 and 100) [5,7]. Across studies and treatment arms, VA improvements ranged from +10.3 to +11.8 ETDRS letters at year one and +9.4 to +11.4 letters at year two [5,7]. In general, DMO patients receiving faricimab achieved greater reductions in CST over time and greater proportions of patients in both faricimab arms achieved absence of intraretinal fluid and absence of DMO (defined as CST <325 µm) through year two compared with aflibercept [1,7].
Over two years, faricimab demonstrated sustained efficacy with up to Q16W dosing. More than half of patients (51.0%–52.8%) in the faricimab T&E arms achieved Q16W dosing at week 52, increasing to ≥60% (60.0%–64.5%) at week 96 [7]. The proportion (pooled) who achieved Q12W or Q16W dosing was 78.1% overall [7]. Treatment with faricimab was well tolerated through study end, and there were no cases of retinal vasculitis or occlusive retinal vasculitis [7].
Taken together, year two data from YOSEMITE and RHINE support the hypothesis that dual inhibition of Ang-2 and VEGF-A with faricimab may promote vascular stability and extend treatment durability beyond current anti-VEGF therapies for DMO, considered David A Eichenbaum, Retina Vitreous Associates of Florida, USA, presenting updated study results at the Royal College of Ophthalmologists 2022 Annual Congress [7].
• Comparative durability in direct comparison with aflibercept T&E?
While efficacy and durability results with faricimab in the phase 3 clinical trials are highly promising, it has yet to be demonstrated whether faricimab would show improved durability in a direct comparison with aflibercept given using the same flexible T&E-based dosing regimen [8].
Year two results from TENAYA and LUCERNE in nAMD, as well as results from extension studies of faricimab in patients with nAMD and DMO, will provide further insights to better understand the potential long-term benefits of dual Ang-2 and VEGF-A inhibition and resulting vascular stability.
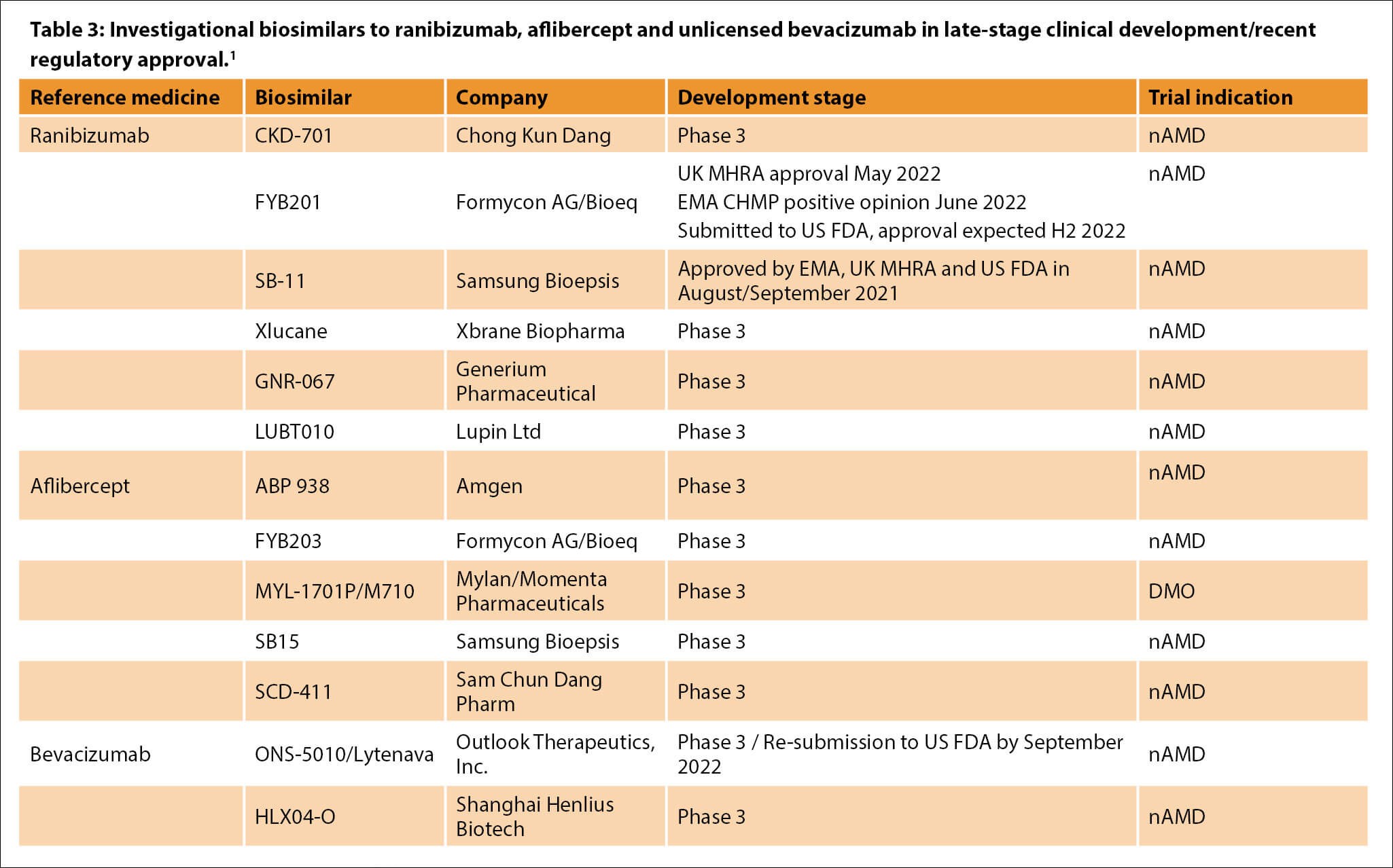
CHMP, Committee for Medicinal Products for Human Use; DMO, diabetic macular oedema; EMA, European Medicines Agency; MHRA, Medicines and Healthcare products Regulatory Agency; nAMD, neovascular age-related macular degeneration; US FDA, United States Food and Drug Administration.
1 Adapted from: Hariprasad SM, et al. etc. Ophthalmol Ther 2022;11(3):959-82.
Biosimilars to established anti-VEGF therapies
Several investigational biosimilars to established intravitreal anti-VEGF therapies are in late-stage development (Table 3) [9,10]. Two ranibizumab biosimilar medicines recently authorised include SB-11 (Byooviz, Samsung Bioepis), approved by European Medicines Agency (EMA), UK MHRA and US Food and Drug Administration (FDA) in 2021, and FYB201 (Ongavia, Midas Pharma GmbH), licensed by the UK MHRA in May 2022 [11–13].
• SB-11: Byooviz
Laboratory studies have shown that the active substance in Byooviz is highly similar to that in ranibizumab in terms of structure, purity and biological activity [11]. The pivotal phase 3 equivalence study compared ranibizumab biosimilar SB-11 and reference ranibizumab product administered every four weeks through week 48 in 705 patients with nAMD.
Equivalence in efficacy was demonstrated for the primary endpoints of change from baseline of optical coherence tomography (OCT) CST at week four and BCVA at week eight, with additional safety data provided through the six-month visit [14]. Predefined equivalence margins for adjusted treatment differences were -36 μm to 36 μm for CST and -3 letters to 3 letters for BCVA. Longer-term results at one year further support the biosimilarity established between SB-11 [15]. At week 52, change from baseline in CST was −140.0 μm and −125.1 μm and change from baseline in BCVA was +9.8 letters and +10.4 letters for SB-11 and ranibizumab, respectively [15].
For the FDA, the primary endpoint measure was change from baseline in BCVA at week eight, while for the EMA the primary endpoint was change from baseline in CST at week four [14].
• FYB201: Ongavia/Ranivisio
The phase 3 COLUMBUS-AMD study evaluated the clinical equivalence of proposed biosimilar FYB201 (Ongavia/Ranivisio, Midas Pharma GmbH) and reference ranibizumab in patients with treatment-naÏve, subfoveal CNV caused by nAMD [16]. Both treatments were given every four weeks through 48 weeks. Results demonstrated the equivalence of FYB201 and reference ranibizumab in terms of efficacy, safety, and immunogenicity in patients with nAMD.
Mean improvement in BCVA from baseline was +5.1 for FYB201 and +5.6 ETDRS letters for reference ranibizumab at week eight. Least squares mean difference for the change from baseline between FYB201 and reference ranibizumab was -0.4 ETDRS letters (90% confidence interval [CI], -1.6 to 0.9). Primary endpoint was met as the 90% CI was within the predefined equivalence margin of -3.5 to 3.5 ETDRS letters.
In June 2022, the CHMP issued a positive opinion for FYB201 (Ranivisio, Midas Pharma GmbH), intended for the treatment of nAMD, visual impairment due to macular oedema or CNV, and visual impairment due to proliferative diabetic retinopathy. Data show that Ranivisio has comparable quality, safety and efficacy to reference drug ranibizumab in patients with nAMD, noted CHMP.
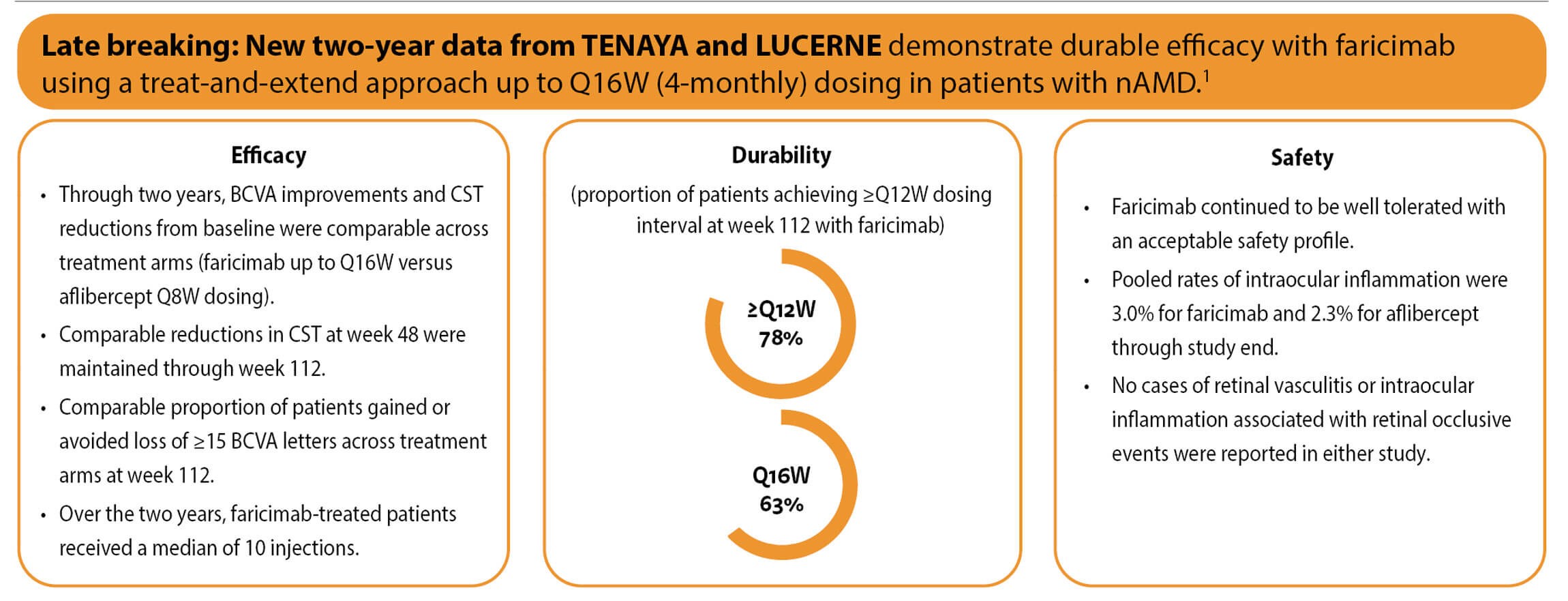
BCVA, best-corrected visual acuity; CST, central subfield thickness; nAMD, neovascular age-related macular degeneration; Q8W, every 8 weeks; ≥Q12W, every 12 weeks or longer; Q16W, every 16 weeks.
1 Khanani A. Faricimab in neovascular age-related macular degeneration: Year 2 efficacy, safety, and durability results from the phase 3 TENAYA and LUCERNE trials. Presentation at the American Society of Retina Specialists Annual Meeting, New York City, USA, 14 July 2022.
References
1. Vabysmo 120 mg/mL solution for injection [Summary of Product Characteristics]; Roche Products Limited, UK, May 2022.
2. National Institute for Health and Care Excellence. Faricimab for treating wet age-related macular degeneration (TA800). Technology appraisal guidance. 29 June 2022.
www.nice.org.uk/guidance/ta800
3. National Institute for Health and Care Excellence. Faricimab for treating diabetic macular oedema (TA799). Technology appraisal guidance.
www.nice.org.uk/guidance/ta799
Last accessed June 2022.
4. Heier JS, Khanani AM, Quezada Ruiz C, et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022;399(10326):729-40.
5. Wykoff CC, Abreu F, Adamis AP, et al. Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet 2022;399(10326):741-55.
6. Eichenbaum DA. Faricimab in neovascular age-related macular degeneration: updated efficacy, safety, and durability in the phase 3 TENAYA and LUCERNE trials. Presentation at the Royal College of Ophthalmologists Annual Congress, Glasgow, UK and Virtual; 23–26 May 2022.
7. Eichenbaum DA. Efficacy, durability, and safety of faricimab in diabetic macular oedema: 2-year results from the phase 3 YOSEMITE and RHINE trials. Presentation at the Royal College of Ophthalmologists Annual Congress, Glasgow, UK and Virtual; 23–26 May 2022.
8. Chia MA, Keane PA. Beyond anti-VEGF: can faricimab reduce treatment burden for retinal disease? Lancet 2022;399(10326):697-9.
9. Hariprasad SM, Gale RP, Weng CY, et al. An introduction to biosimilars for the treatment of retinal diseases: a narrative review. Ophthalmol Ther 2022;11(3):959-82.
10. Sharma A, Kumar N, Parachuri N, et al. Biosimilars for retinal diseases: an update. Am J Ophthalmol 2021;224:36-42.
11. European Medicines Agency. Byooviz (ranibizumab): medicine overview. EMA/446502/2021. Last updated August 2021.
12. Byooviz 10 mg/ml solution for injection [Summary of Product Characteristics]; Samsung Bioepis UK Limited, UK, first authorised August 2021.
13. Ongavia 10 mg/ml solution for injection [Summary of Product Characteristics]; Midas Pharma GmbH, Germany, May 2022.
14. Woo SJ, Veith M, Hamouz J, et al. Efficacy and safety of a proposed ranibizumab biosimilar product vs a reference ranibizumab product for patients with neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol 2021;139(1):68-76.
15. Bressler NM, Veith M, Hamouz J, et al. Biosimilar SB11 versus reference ranibizumab in neovascular age-related macular degeneration: 1-year phase III randomised clinical trial outcomes. Br J Ophthalmol 2021 Epub ahead of print.
16. Holz FG, Oleksy P, Ricci F, et al. Efficacy and safety of biosimilar FYB201 compared with ranibizumab in neovascular age-related macular degeneration. Ophthalmology 2022;129(1):54-63.
17. European Medicines Agency and the European Commission. Biosimilars in the EU: information guide for healthcare professionals.
https://www.ema.europa.eu/en/documents/
leaflet/biosimilars-eu-information-guide
-healthcare-professionals_en.pdf
18. US Food and Drug Administration. Biosimilar and interchangeable products. Updated October 23, 2017.
https://www.fda.gov/drugs/
biosimilars/biosimilar-and
-interchangeable-products.
19. Medicines and Healthcare products Regulatory Agency. Guidance on the licensing of biosimilar products. 6 May 2021.
https://www.gov.uk/government/
publications/guidance-on-the
-licensing-of-biosimilar-products
20. National Institute for Health and Care Excellence. NICE’s biosimilars position statement. August 2016.
https://www.nice.org.uk/Media/Default/
About/what-we-do/NICE-guidance/NICE
-technology-appraisals/Biosimilar-medicines
-postition-statement-aug-16.pdf
21. AMGEN. 2021 Biosimilars Trends Report.
https://www.amgenbiosimilars.com/
commitment/trends-report
[All links last accessed June 2022].
COMMENTS ARE WELCOME






