Aetiology of postoperative refractive surprise
Weber coined the term “wrong eye, wrong intraocular lens, wrong patient” in 2008 as an aide memoir of major factors believed to underlie refractive surprise – defined as a significant unintended difference between dioptric refraction following cataract surgery, with the preoperative refractive aim [1-3]. Between 1998 and 2018, at least 21 relevant papers have been published on refractive surprise, revealing that its currently understood risk factors are better grouped into those that already affect the patient, those that arise in preoperative planning and those that arise during surgery.
Underlying corneal pathology, previous ophthalmic surgery and significant preoperative refractive error reduce the accuracy of formulae commonly used to select the lens for implantation after routine phacoemulsification. The same logic is applicable to paediatric patients, whose eyes grow at a variable rate into teenage years [4-9]. Inaccurate preoperative biometry is a major factor in the development of postoperative refractive surprise. Manufacturers recommend lens’ A constants based on ultrasound biometry, which can differ from clinical settings where optical partial coherence interferometry is common. Serial modification has been shown to successfully reduce unexpected refractive error when using the latter measuring system [10]. In the past half-century, formulae used to predict lens power for an optimal postoperative refractive state have evolved.
"If surgeons cannot determine the probability of whether IOLs truly lie at their nominal power instead of elsewhere within pre-specified ranges, then manufacturers are imposing a rate-limiting step on the improvement of precision of postoperative refractive outcomes"
Recent years have brought the development of formulae that incorporate machine learning and regression analysis to work on very large datasets, to better estimate the artificial effective lens position. Absolute error values are used to compare new formulae [9,11]. Combined ophthalmic procedures, intraoperative complications such as vitreous loss, capsular rupture and retained viscoelastic material are all risk factors for altering the refractive error of the eye postoperatively [4,5,11]. However, Bryant et al. reported conflicting data showing that surgical skill was not an independent risk factor for increased refractive error. Other minor causes of refractive surprise arise from various sources of human error. Poor theatre staff communication and coordination allow rare mistakes, including the selection of the incorrect intraocular lens (IOL) and operating on the incorrect eye [8]. Four previous case reports have also highlighted incorrect power labels on lens packaging. These incidents require the lens to be removed for the manufacturer to confirm the error [1,13,14,15].
Regardless of improved understanding of the factors influencing refractive surprise, and advances in the mathematical formulae to select the most accurate IOLs, significant refractive surprises persist. An often forgotten factor in postoperative refractive surprise is the accuracy of the nominal dioptric labels on IOLs themselves. An informal poll at the British Society for Refractive Surgery 2022 meeting showed a widespread lack of awareness of current industry standards for tolerances applied to IOL powers. To compare to junior training grades, the same informal poll was applied to the Belfast Eye Conference 2022, targeted at trainee doctors, showing a comparable lack of awareness.
The standards for IOL production
The British Standard implements EN ISO 11979-2:2014, which defines the acceptable ranges for nominal dioptric powers of manufactured IOLs. This standard supersedes the 1999 version, and states that its users (IOL manufacturing companies) are responsible for its correct application. In this article, ISO 11979 refers to the 2014 version [16].
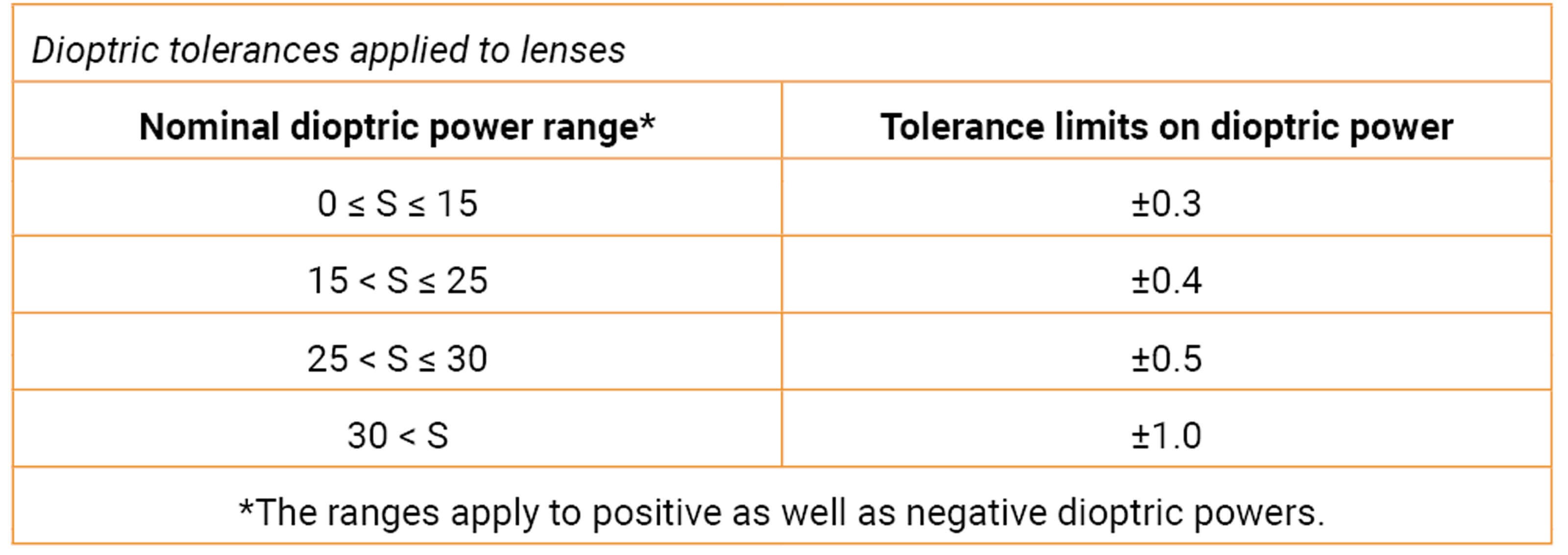
Table 1: Tolerance limits that apply to nominal dioptric IOL powers within different ranges
ISO 11979 sets the standard for the powers of all IOLs – spherical, aspheric, monofocal, toric, multifocal, accommodative and phakic. For in situ conditions, Table 1 (BSI Standards Limited) shows the limits that apply to either spherical or aspheric monofocal IOLs. There are various methods of calculating dioptric power, which have respective assumptions. For example, when using measured dimensions of IOLs, there is assumed exact alignment between the front and back IOL surfaces along the optical axis. This is clearly not the case during and possibly following lens manipulation for implantation. To summarise the overall effect of these tolerances, a labelled +31 Dioptre lens may in reality be anything from a +30.01 to +31.99 D in actual power, causing a very significant refractive surprise.
Additionally, ISO 11979 suggests methods for manufacturers to demonstrate that their IOLs satisfy the standard, with provided cut-off values. However, manufacturers are able to select their own methods. All methods make use of model eyes, also recommended by ISO 11979. These are designed to mimic the human cornea’s physiological spherical aberration, though remain limited in this regard due to an oversimplification and standardisation of corneal anatomy [17,18].
Investigating standard maintenance with IOL manufacturing companies
Leading IOL manufacturers were identified in the Europe Intraocular Lens Market Research report for 2021-27 [19]. Six of the 13 included in the report were considered most relevant to the NHS: Johnson & Johnson, Bausch and Lomb, Hoya, Rayner, Alcon and Lenstec, Inc. Specific popular lenses from each company were chosen as a focus (Table 2), for up to four contact attempts between 19 February and 19 May 2022.
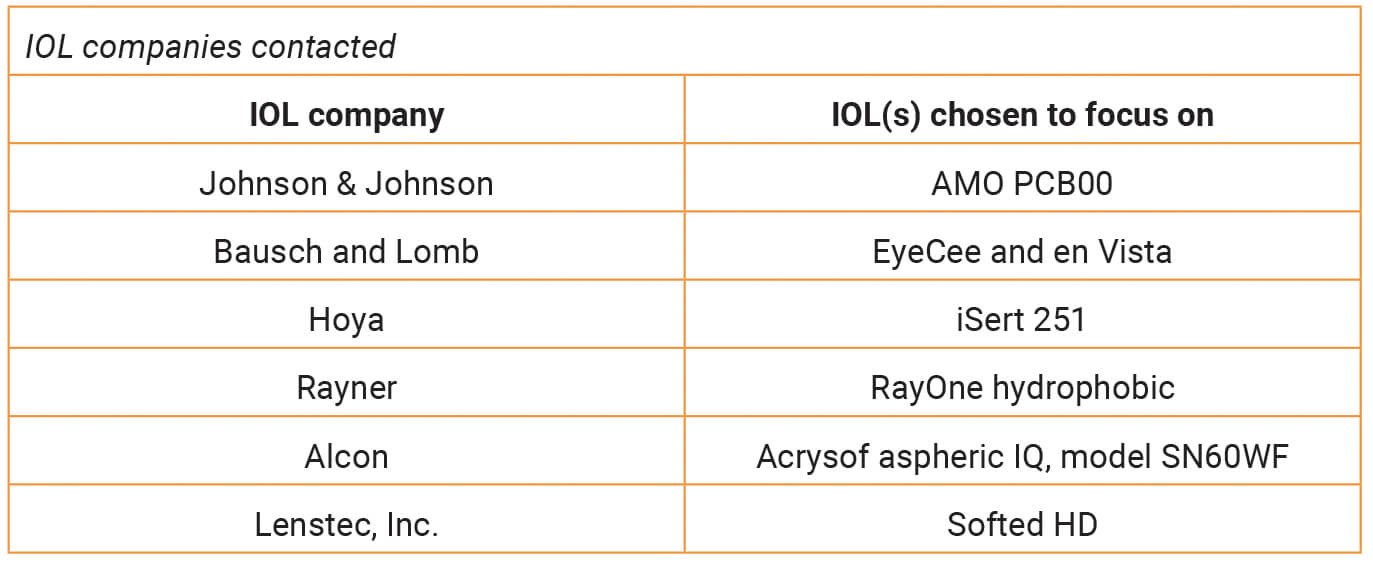
Table 2: Showing the lens models chosen to contact IOL companies about.
Each company was asked about their methods of determining and guaranteeing power tolerance limits on their IOLs. Four did not provide any information within the three months of trying to get in touch. Of the two that did:
Alcon reported that the dioptric power tolerance of IOL model SN60WF satisfies the standard power tolerance. Further details about methods of power verification were identified as proprietary confidential information.
Lenstec, Inc. set up a meeting to discuss the standard. The Food and Drug Administration (FDA) requires them to reduce the relevant power tolerances in the development of IOLs with quarter-dioptre intervals rather than the half-dioptre intervals on the market. This is not reflected in the published standard. Given that there is currently no literature addressing the verification of dioptric tolerances of IOLs that are beyond the 10-30 D range, tolerances applied to them are based on extrapolated data. Additionally, there is no readily available distribution of where manufactured IOLs actually lie within tolerances around their nominal powers; the true power of IOLs represents a function of the IOL and the measuring system employed. Both of these contribute inherent levels of error. Different methods of IOL production also have varying levels of inherent error.
Discussion
These discussions, and the lack thereof, with IOL manufacturers revealed largely that the nominal, labelled powers on IOLs do not necessarily represent their true powers. Manufacturers are not currently willing to share their methods of verifying that their IOLs are within the standard power tolerances. Noting that different methods between manufacturing companies will indicate varying levels of accuracy, there is likely a perception that cataract and refractive surgeons – as the consumer market of IOLs – will make independent judgements on their corporate reliability. This is not a new concept. Antičić et al. suggested that potential reputational damage to manufacturer reliability may cause under-reporting of IOL power mislabelling [15]. If IOL manufacturers want to stand out from their competitors, their own tolerances and levels of precision in measuring dioptric power should be published as important markers of manufacturing quality. An improvement on this would be labelling actual powers on IOLs.
Further work is still yet to be done. It is unclear whether there is discrepancy in standard application when manufacturing factories, head offices and consumer markets are in countries outside of Europe (thus outside of the standard’s jurisdiction). There should be improved awareness of the standard within the ophthalmology training pathway and among consultants. Ideally, surgeons should be able to access any information from manufacturers that directly influences achieving accurate refractive aims after cataract surgery. Current focuses on improving IOL selection formulae using artificial intelligence and advanced computational methods, may improve the precision of refractive outcomes by incremental degrees. However, IOLs themselves are likely responsible for a significant proportion of larger refractive surprises. If the lack of knowledge regarding the accuracy of power continues, then surgeons are at a loss – they are effectively unable to determine the probability of whether IOLs are actually at their nominal powers.
TAKE HOME MESSAGES
-
A very significant cause of refractive surprise across cataract surgery lists is likely to be the IOLs themselves.
-
There is an industry-imposed standard for the limits in which nominal powers of IOLs actually lie within.
-
These limits vary depending on the nominal power of the lens.
-
IOL manufacturing companies classify their methods of maintaining this standard as proprietary confidential information.
-
Different methods of maintaining this standard imply different degrees of accuracy in producing IOLs that actually lie at their nominal powers.
References
1. Kohnen S. Postoperative refractive error resulting from incorrectly labelled intraocular lens power. J Cataract Refract Surg 2000;26(5):777-8.
2. Carlson AN, Stewart WC, Tso PC. Intraocular lens complications requiring removal or exchange. Surv Ophthalmol 1998;42(5):417-40.
3. Weber P. Wrong eye, wrong IOL, wrong patient. OMIC Digest 2008;18(3):1-4.
4. Geiger M, Patnaik J, Miller DC, et al. Ocular comorbidities as risk factors for refractive surprise following cataract surgery. ARVO Annual Meeting 2019;60(9):2072.
5. Lundström M, Dickman M, Henry Y, et al. Risk factors for refractive error after cataract surgery: Analysis of 282,811 cataract extractions reported to the European Registry of Quality Outcomes for cataract and refractive surgery. J Cataract Refract Surg 2018;44(4):447-52.
6. Ho VWM, Stanojcic N, O’Brart NAL, O’Brart DPS. Refractive surprise after routine cataract surgery with multifocal IOLs attributable to corneal epithelial basement membrane dystrophy. J Cataract Refract Surg 2019;45(5):685-9.
7. Gupta OK, Drinkwater OJ, VanDusen KW, et al. Prevalence of ocular surface dysfunction in patients presenting for cataract surgery evaluation. J Cataract Refract Surg 2018;44(9):1090-6.
8. Bryant T, Farivari N, Fan K, et al. Factors associated with refractive surprise after cataract surgery. Invest Ophthalmol Vis Sci 2016;57(12):1984.
9. Xia T, Martinez CE, Tsai LM. Update on intraocular lens formulas and calculations. Asia Pac J Ophthalmol (Philia) 2020;9(3):186-93.
10. Madge SN, Khong CH, Lamont M, et al. Optimization of biometry for intraocular lens implantation using the Zeiss IOLMaster. Acta Ophthalmol Scand 2005;83(4):436-8.
11. McCartney PJ. Refractive surprise after piggyback intraocular lens implantation (letter). J Cataract Refract Surg 2011;37(7):1372-3.
12. Steeples LR, Hingorani M, Flanagan D, Kelly SP. Wrong intraocular lens events – what lessons have we learned? A review of incidents reported to the National Reporting and Learning System: 2010-4 versus 2003-10. Eye 2016;30(8):1049-55.
13. Solebo LA, Walker RJE, Dabbagh A. Intraocular lens exchange for pseudophakic refractive surprise due to incorrectly labelled intraocular lens. J Cataract Refract Surg 2012;38(12):2197-8.
14. Ravi K, Senthil S, Pesala V. Refractive surprise following implantation of correct powered intraocular lens – a real surprise! Int Ophthalmol 2012;32(6):603-5.
15. Antičić M, Ardjomand N, Sarny S, et al. “Numbers sometimes lie” – refractive surprise following IOL mislabeling by the manufacturer. Eye 2019;33(6):868-70.
16. Technical committee ISO/TC 172, Technical committee CEN/TC 170. Ophthalmic implants – intraocular lenses Part 2: Optical properties and test methods. BS EN ISO 11979-2:2014.
https://infostore.saiglobal.com/
preview/98702525335.pdf?sku=869293
_SAIG_NSAI_NSAI_2067137
Last accessed August 2022.
17. Norrby S, Piers P, Campbell C, van der Mooren M. Model eyes for evaluation of intraocular lenses. Appl Opt 2007;46(26):6595-605.
18. Wang L, Dai E, Kock DD, Nathoo A. Optical aberrations of the human anterior cornea. J Cataract Refract Surg 2003;29(8):1514-21.
19. Europe intraocular lens market size, share & industry trends analysis report by end-use, by product (multifocal, monofocal, toric, and accommodative), by country and growth forecast, 2021-2027. Knowledge based value research 2022.
https://www.mordorintelligence.com/
industry-reports/europe-intra
-ocular-lens-market-industry
Last accessed August 2022.
Note: Permission to reproduce extracts from British Standards is granted by BSI Standards Limited (BSI). No other use of this material is permitted. British Standards can be obtained from BSI Knowledge: knowledge.bsigroup.com
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME