The authors review the current treatment options for this condition.
Radiation retinopathy (RR) occurs as a complication after exposure to any type of radiation (external beam, plaque brachytherapy and stereotactic radiosurgery) in the orbital or adnexal region. These include nasopharyngeal and paranasal sinus tumours, or orbital tumours such as choroidal melanomas, retinoblastomas and choroidal metastasis. Radiation is also used to treat benign conditions such as in thyroid ophthalmopathy, exudative inflammatory processes of the posterior segment, age-related macular degeneration and choroidal haemangioma.
It was first described in 1933 by Stallard and has been seen in several case reports. According to a number of case reports, the most common cause of radiation retinopathy in the UK appears to be external beam therapy [1]. Radiation retinopathy is often dose, daily fraction size and fraction interval dependent, with the usual threshold dose for retinal damage at 30-35Gy [1].
The time of onset varies greatly and can occur between one month to 15 years after exposure, but most commonly occurs between six months and three years. A recent retrospective study reported an incidence of proliferative radiation retinopathy of 5.8% at five years, 7% at 10 and 15 years in 3841 of the eyes treated with plaque radiotherapy for uveal melanoma [2].
In addition to retinopathy, radiation exposure can also result in radiation dermatitis and skin atrophy in the periocular region, xerophthalmia, corneal and scleral dystrophy and necrosis, iridocyclitis, lacrimal system stenosis and damage, optic neuropathy, cataract, vitreous haemorrhage and retinal detachment [3].
Pathophysiology
Radiation causes microangiopathy of small retinal vessels secondary to endothelial cell loss and capillary closure in the posterior segment, which is more sensitive than the peripheral retina. A preferential loss of vascular endothelial cells and sparing of pericytes differentiates it from diabetic retinopathy. The hypothesised pathophysiology is due to direct exposure of endothelial cells to high ambient oxygen and iron found in the blood, which generates free radicals, leading to cell membrane damage [4-5]. Other clinical signs of RR include macular oedema, microaneurysms, telangiectasias, neovascularisation, rubeosis, neovascularisation of the retina, vitreous haemorrhage and tractional retinal detachment [1].
Risk factors
The main risk factor for developing radiation retinopathy is a higher total radiation dose with incidence significantly increased at doses greater than 45Gy [6]. Further, hyper-fractionation was shown to be associated with a decreased incidence of radiation retinopathy, and patients who received less than 25Gy in fractions of 2Gy or less are also unlikely to develop clinically significant retinopathy [7]. Patients with pre-existing vascular diseases, including diabetes, hypertension or previous chemotherapy have been reported to develop radiation retinopathy at a lower threshold [8].
Diagnosis
History
Symptoms are dependent on stage of retinopathy. Patients with early or mild retinopathy may be asymptomatic initially. However, with late progression, patients can report blurred vision or floaters [9].
Differential diagnosis
Radiation retinopathy is mainly distinguished from its differentials (Table 1) from the history, particularly with identified exposure to ionising radiation. Systemic vascular risk factors can be assessed with basic investigations such as blood pressure readings, blood tests including full blood count with antibody, vasculitis screen, electrolyte panel, lipid screen and random blood glucose. Other investigations such as a carotid doppler ultrasound or a thorough history taking of past medical history, particularly blood disorders, drug history and social history such as a positive history of smoking are also pertinent to diagnosis [10].
Table 1: Differential diagnosis of radiation retinopathy [9].
Diabetic retinopathy
Branch / Central retinal vein occlusion
Ocular ischaemic syndrome
Hypertensive retinopathy
Coat’s disease
Perifoveal telangiectasia
In RR there is atrophy of the retinal pigment epithelium (RPE) and also less microaneurysms presented compared to diabetic retinopathy or other retinal vasculopathies. Additionally, neovascularisation does not typically extend through the internal limiting membrane into the vitreous. Telangiectatic vessels can also be present in radiation retinopathy but this is rarely the only feature seen and thus differentiates it from perifoveal telangiectasia [11-12].
Investigations
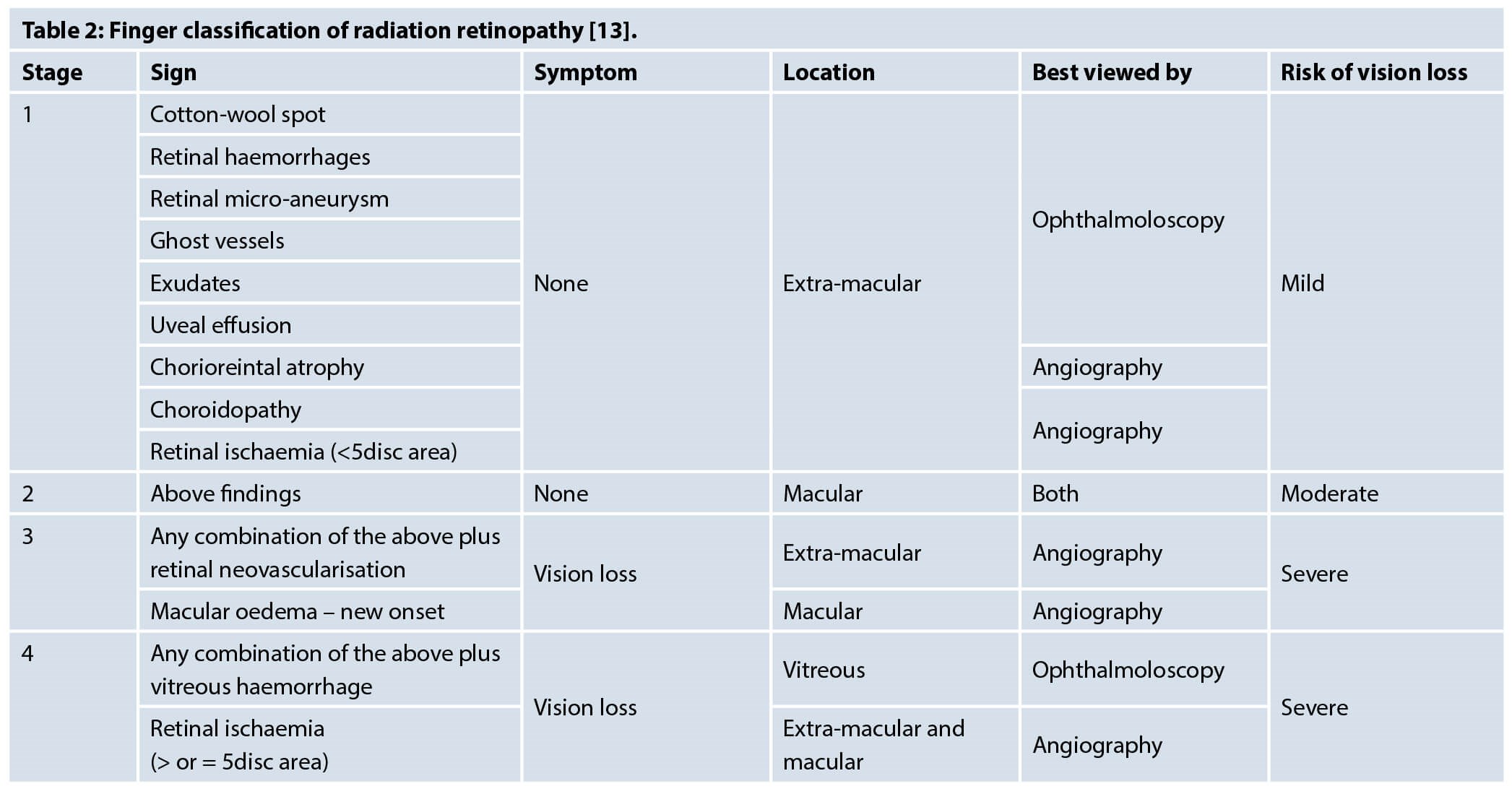
Ophthalmoscopy is used to look for common findings such as cotton wool spots, retinal haemorrhages, ghost vessels and exudates. Retinal microaneurysms and uveal effusions are seen infrequently, whereas retinal neovascularisation, vitreous haemorrhage and tractional detachment are signs that indicate significant risk of visual loss [13]. Fluorescein angiogram can help detect the microvascular presentations and indocyanine green angiography can show precapillary arteriolar occlusion and areas of choroidal hypoperfusion. Optical coherence tomography (OCT) can also help detect macular oedema [9]. Finger et al. published a classification of radiation retinopathy to help predict prognosis of the condition (Table 2) [13].
Management
There are currently no National Institute of Health & Care Excellence (NICE) established treatment guidelines for RR. As there are many clinical similarities between RR and diabetic retinopathy, treatment that is presently used follows a similar therapeutic principle, which is to reduce macular oedema and prevent neovascularisation. Common treatment modalities include the use of laser photocoagulation, corticosteroids and anti-vascular endothelial growth factor (anti-VEGF) agents, such as Bevacizumab and Ranibizumab [14-19, 22-26].
Treatment decision should be made in conjunction with visual prognosis, in particular, the presence of macular ischaemia. Minor degrees of retinopathy such as subtle capillary fallout or occasional dilated channels and micro-aneurysms may not affect vision and may remain stable for years. Thus, no treatment might be necessary but patients might require annual follow-up as the disease is slowly progressive [20].
Retinal laser photocoagulation
Laser photocoagulation can be used as focal therapy for macular oedema and exudation, or scattered / pan photocoagulation in controlling neovascularisation. Fovea involving macular oedema remains the major cause of visual loss in patients with RR. Hykin et al. reported 42% of the affected eyes had an improvement of one line on Snellen chart at six months when compared to the observational group, but the difference at 12 and 24 months was not significant. Axer-Siegel et al. also demonstrated improvement of retinal thickness and resolution of exudates with focal laser, but visual outcome only improved marginally [21].
“Radiation retinopathy is often dose, daily fraction size and fraction interval dependent, with the usual threshold dose for retinal damage at 30-35Gy”
Pan-retinal photocoagulation (PRP) is used as the treatment for many forms of ischaemic retinopathies and might be used in patients with high risk characteristics for vision loss such as neovascularisation of optic disc and vitreous haemorrhage. Reports in the literature have shown PRP to cause regression of neovascularisation in RR. This is achieved by targeting area of ischaemia through angiography [14]. In a study of 45 patients, Finger et al. found that sector argon laser photocoagulation regressed clinically evident RR in 64.4% patients with early onset retinopathy. However, a third of this group still sustained a loss of three or more lines in vision due to radiation maculopathy [13].
For prophylactic photocoagulation, the evidence remains unclear and long-term data is required.
Photodynamic therapy
Photodynamic therapy (PDT) is mainly used to cause regression of neovascularisation. It is comprised of a two-step process in which an intravenous infusion of verteporfin is followed by irradiance with a 689nm laser for around 83 seconds. Verteporfin binds to serum lipoproteins which bound favourably to choroidal new vessels. PDT induces formation of toxic oxygen species that leads to subsequent thrombosis of these new vessels. Several case studies have reported improved visual acuity after PDT. However, the use of photodynamic therapy has been insufficiently investigated for long-term prognosis and more evidence is required in terms of efficiency and safety [14].
Corticosteroids
Intravitreal corticosteroid injections are used to reduce radiation-induced macular oedema. Specifically, the use of triamcinolone acetonide has been reported to help achieve visual stability by Shields et al. [21]. Intravitreal dexamethasone implants have also been reported to reduce macular oedema. Some authors proposed that steroid reduces the osmotic swelling of Muller’s cell, while others suggested that it stabilises endothelial tight junctions and therefore reduces leukocyte migration [21]. Due to the associated side-effects of cataracts and raised intraocular pressure, alternate treatment should be considered for younger patients and those at risk of glaucoma.
Horgan et al. published more recently a randomised, controlled trial looking at periocular injection of triamcinolone for prevention of macular oedema. Periocular triamcinolone was given at the time of 125I plaque application with patients with uveal melanoma. This has shown some promising results in reducing risk of macular oedema up to 18 months after plaque radiotherapy [15].
Anti-VEGF agents
Humanised monoclonal anti-vascular endothelial growth factor (VEGF) antibody has been shown to help improve macular oedema and to reduce intraocular neovascularisation. Studies have shown varied improvement in visual acuity, either as single injections or multiple treatments [16-18]. The variation is hypothesised to be due to age and duration of macular oedema prior to treatment, with an older age and long-standing oedema associated with reduced treatment effect. A study demonstrated that a single injection of Bevacizumab reduced mean foveal thickness and improved mean visual acuity six weeks after injection in patients with macular oedema. However, this was not sustained and four months following injection, mean visual acuity (VA) returned to pre-treatment levels with an increase in foveal thickness [22]. Another study demonstrated that injections of Bevacizumab every six to eight weeks following diagnosis of retinopathy improved or stabilised visual acuity in all treated patients with a mean follow-up of 4.7 months following first injection. Continued treatment up to 18 months also helped improve and achieve stability in visual acuity in the majority of patients [23].
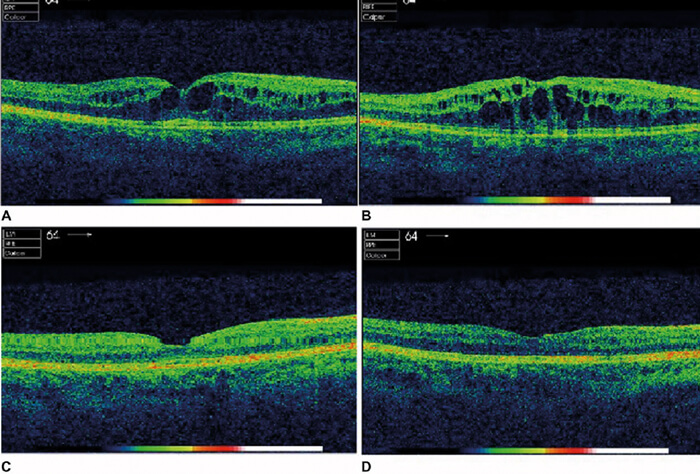
Figure 1: OCT results initially after radiation and subsequently after injections. (A) Before first treatment in January 2016. (B) Before second treatment in June 2016. (C) After second intravitreal Eylea in August 2016. (D) After third intravitreal Eylea in September 2016.
There are currently no published clinical trials to compare different commercially available anti-VEGF. Ranibizumab was used in a patient with radiation maculopathy with previous tachyphylaxis to Bevacizumab and showed excellent response [24]. In a different case report, Aflibercept showed an improvement on OCT macular thickness (Figure 1) and VA improved from 6/18 to 6/15 after a total of three intravitreal injections [19].
“Studies have demonstrated the effectiveness of anti-VEGF therapy in reducing radiation-related neovascularisation”
Moreover, studies have demonstrated the effectiveness of anti-VEGF therapy in reducing radiation-related neovascularisation. A study reported a more rapid improvement in neovascularisation following Bevacizumab therapy compared with PRP treatment [25]. However, the effectiveness remains debatable, as another study reported that Bevacizumab reduced iris neovascularisation following proton beam irradiation but this was not sustained and recurred within 12 months [26].
Other therapies
Alternative treatment modalities have been reported in the literature to treat various causes of RR with mixed results and are not used extensively.
Hyperbaric oxygen therapy (HBOT) improves oxygenation and is hypothesised to counteract hypoxic cells in radiation induced vasculopathies and neuropathies. One case report has shown improvement of clinical picture in terms of retinal exudates and visual fields following HBOT, however, visual acuity did not significantly improve [27].
Oral pentoxifylline is used as a treatment for peripheral vascular disease by causing vasodilation, decreasing blood viscosity and therefore improving blood flow and oxygenation. A report by Gupta et al. has shown improved capillary perfusion and significant improvement of vision following eight months of oral treatment [28].
Conclusion
Radiation retinopathy causes significant visual morbidity following radiation to the head and neck region due to macular oedema and the risk of choroidal neovascular membranes. Current treatment which aims to target this includes laser photocoagulation, corticosteroid and anti-VEGF injections and have reported varying degrees of success. Decision on which treatment to use also depends on the patient’s lens status, risk factors of glaucoma and their visual prognosis. More effort recently has been put in to investigate early treatment in preventing radiation retinopathy.
References
1. Amoaku WM, Archer DB. Fluorescein angiographic features, natural course and treatment of radiation retinopathy. Eye 1990;4:657-67.
2. Bianciotto C, Shields C, Pirondini C, et al. Proliferative Radiation retinopathy after plaque radiotherapy for uveal melanoma. Ophthalmology 2010;117(5):1005-12.
3. Abramson DH, Beaverson KL, Chang ST, et al. Outcome following initial external beam radiotherapy in patients with Reese−Ellsworth Group Vb retinoblastoma. Arch Ophthalmol 2004;122:1316-23.
4. Archer DB, Gardiner TA. Ionizing radiation and the retina. Curr Opin Ophthalmol 1994;5(3):59-65.
5. Giuliari GP, Sadaka A, Hinkle DM, et al. Current treatments for radiation retinopathy. Acta Oncol 2011;50:6-13.
6. Parsons JT, Bova FJ, Fitzgerald CR, et al. Radiation retinopathy after external-beam irradiation: analysis of time-dose factors. Int J Radiat Oncol Biol Phys 1994;30(4):765-73.
7. Monroe AT, Bhandare N, Morris CG, Mendenhall WM. Preventing radiation retinopathy with hyperfractionation. Int J Radiat Oncol Biol Phys 2005;61(3):856-64.
8. Gupta A, Dhawahir-Scala F, Smith A, et al. Radiation Retinopathy: Case report and review. BMC Ophthalmol 2007;7:6.
9. Yanoff M, Duker JS, Augsburger JJ: Ophthalmology. 2nd ed. St Louis, MO: Mosby; 2004.
10. Raja V, Rajagopalan S, Kashab T, Moriarty B. Radiation retinopathy: a mistaken diagnosis of hypertensive retinopathy. Clin Exp Optom 2007;90:468‑70.
11. Zamber RW, Kinyoun JL. Radiation Retinopathy. West J Med 1992;157:530-3.
12. Viebahn M, Barricks ME, Osterloh MD. Synergism between diabetic and radiation retinopathy: case report and review. BJO 1991;75:629-32.
13. Finger PT, Kurli M. Laser photocoagulation for radiation retinopathy after ophthalmic plaque radiation therapy. Brit J Ophthalmol 2005;89:730-8.
14. Giuliari G, Sadaka A, Hinkle D, Simpson E. Current treatments for radiation retinopathy. Acta Oncologica 2010;50(1):6-13.
15. Horgan N, Shields CL, Mashayekhi A, et al. Periocular triamcinolone for prevention of macular edema after plaque radiotherapy of uveal melanoma: A randomized controlled trial. Ophthalmol 2009;116:1383-90.
16. Finger PT, Chin K. Anti-vascular endothelial growth factor bevacizumab (avastin) for radiation retinopathy. Arch Ophthalmol 2007;125(6):751-6.
17. Finger PT. Radiation retinopathy is treatable with anti-vascular endothelial growth factor bevacizumab (Avastin). Int J Radiat Oncol Biol Phys 2008;70(4):974-7.
18. Gupta A, Muecke JS. Treatment of radiation maculopathy with intravitreal injection of bevacizumab (Avastin). Retina 2008;28(7):964-8.
19. Pooprasert P, Young-Zvandasara T, Al-bermani A. Radiation retinopathy treated successfully with aflibercept. BMJ Case Reports 2017:bcr-2017-220744.
20. Yanoff M, Duker J: Ophthalmology. Edinburgh: Mosby; 2008.
21. Reichstein D. Current treatments and preventive strategies for radiation retinopathy. Curr Opin Ophthalmol 2015;26:157-66.
22. Mason JO 3rd, Albert MA Jr, Persaud TO, Vail RS. Intravitreal bevacizumab treatment for radiation macular edema after plaque radiotherapy for choroidal melanoma. Retina 2007;27:903-7.
23. Finger PT. Radiation retinopathy is treatable with antivascular endothelial growth factor bevacizumab (Avastin). Int J Radiat Oncol Biol Phys 2008;70:974-7.
24. Jutley G, Shona OA, Leen RC, et al. Response to ranibizumab following tachyphylaxis to bevacizumab in a patient with radiation maculopathy following stereotactic fractionated radiotherapy for optic nerve meningioma. Arch Ophthalmol 2012;130:1466-70.
25. Arriola-Villalobos P, Donate-Lo´pez J, Calvo-Gonza´lez C, et al. Intravitreal bevacizumab (Avastin) for radiation retinopathy neovascularization. Acta Ophthalmol 2008;86:115‑6.
26. Yeung SN, Paton KE, Waite C, Maberley DA. Intravitreal bevacizumab for iris neovascularization following proton beam irradiation for choroidal melanoma. Can J Ophthalmol 2010;45:269-73.
27. Gall N, Leiba H, Handzel R, Pe’er J. Severe radiation retinopathy and optic neuropathy after brachytherapy for choroidal melanoma, treated by hyperbaric oxygen. Eye (Lond) 2007;21:1010-2.
28. Gupta P, Meisenberg B, Amin P, Pomeranz HD. Radiation retinopathy: the role of pentoxifylline. Retina 2001;21:545-7.
TAKE HOME MESSAGE
-
Radiation retinopathy occurs as a complication after exposure to any type of radiation.
-
It is often dose, daily fraction size and fraction interval dependent, with the usual threshold dose for retinal damage at 30-35Gy.
-
Diagnosis for radiation retinopathy can be made through a thorough history and a dilated fundoscopic exam to look for pathological signs including retinal haemorrhage, microaneurysm, telangiectasia and macular oedema.
-
Treatment aim is to reduce macular oedema and neovascularisation. Most common treatment modality is the use of retinal laser photocoagulation, corticosteroids and anti-VEGF treatment.
COMMENTS ARE WELCOME







