Abdul Muhyemin Tarin reviews the presentation, pathophysiology and management of this paraneoplastic syndrome.
Case presentation
A 60-year-old hypermetropic female patient presented with several months’ history of painless blurred vision. Visual acuity (VA) was 6/24 and 6/9-1 in right and left eyes respectively. Two months prior to presentation, this patient was diagnosed with small cell lung cancer, after extensive investigation for a chronic productive cough and unexplained weight loss. She had also been a smoker.
On examination, she had moderate vitritis with snowballs in both eyes, bilateral posterior vitreous detachment with few cells in the vitreous, bilateral disc pallor and retinal vascular attenuation. Fundus fluorescein angiography showed bilateral periphlebitis and optic disc leakage (Table 1). Visual fields were markedly constricted. She had normal blood tests and magnetic resonance imaging (MRI) brain and orbits.

A diagnosis of bilateral posterior vitritis was made. She was commenced on oral prednisolone 60mg OD after infective causes had been ruled out by vitreous tap and blood tests, including serum angiotensin converting enzyme (ACE), syphilis serology, serum anti-nuclear antibodies and serum anti-neutrophil cytoplasm antibodies (ANCA). The inflammation improved, but the subjective vision stayed the same. Her computed tomography (CT) and MRI scan was reviewed by neuroradiology, and a compressive optic nerve lesion was ruled out. Electroretinogram (ERG) showed bilaterally reduced amplitudes with grossly abnormal rod and cone responses, more pronounced in the right eye. Full field ERG demonstrated markedly reduced flash responses in both photopic and scotopic conditions. Oscillating potentials (OP) showed minimal flicker responses. In the absence of other clinically evident disease, she was diagnosed with cancer associated retinopathy (CAR)
Introduction
Paraneoplastic syndromes are a group of conditions that occur when a malignancy causes complex systemic symptoms that cannot be explained by local or distant spread of the primary tumour. They are caused by either an abnormal systemic immune response against cancer cells, or by release of chemical signalling molecules from the neoplasm, that can act at distant sites [1].
“Approximately 10-15% of cancer patients eventually develop paraneoplastic syndromes”
Paraneoplastic syndromes can affect the central nervous system and peripheral nervous system, as well as ocular tissue. Ocular paraneoplastic syndromes are generally classified as: cancer associated retinopathy (CAR), melanoma associated retinopathy (MAR) and bilateral diffuse uveal melanocytic proliferation (BDUMP) [1].
The first cases of CAR were detailed in 1976. Sawyer et al. published a case series of three patients with small cell carcinoma of the lung who later developed vision loss, visual field defects, ring-like scotomas and retinal arteriolar narrowing. Biopsy revealed widespread retinal degeneration [2]. In 1982, antiretinal antibodies were first discovered in cancer patients by Kornguth et al. [3]. Recoverin, a key component in retinal photoreceptors, was discovered in 1992 [4]. Since then, it has been widely established that many different types of malignancies can cause CAR, and over 18 different retinal antigens have been implicated.
Bergson and associates reported the first case of MAR in 1988. Immunofluorescence testing revealed autoantibodies against a specific melanoma antigen, which cross-reacted with retinal bipolar cells and resulted in a similar clinical phenotype as CAR [5]. BDUMP is a third type of ocular paraneoplastic syndrome that is poorly understood, and is characterised by infiltration of the choroid by benign-appearing melanocytes [6].
This article will aim to outline the epidemiology, pathophysiology, diagnosis and treatment of CAR syndromes.
Epidemiology
Approximately 10-15% of cancer patients eventually develop paraneoplastic syndromes [7]. CAR affects men and women equally, and is common amongst middle-aged to older patients. One study demonstrated an age range between 37 and 76 years, with 22 out of 28 patients being above 60 years of age. Smokers are at an increased risk of CAR, which is explained by the majority of patients having lung cancer. The most common malignancy that leads to CAR is small cell cancer of the lung, which affects about 60% of cases. Other reported malignancies include: non-small cell lung cancer, endometrial carcinoma, colon cancer, embryonal rhabdomyosarcoma, adeno-squamous carcinoma of the pancreas, and squamous cell carcinoma of the vocal cords [8].
Pathophysiology
Recoverin is the most investigated retinal protein involved in the pathogenesis of autoimmune retinopathy [7]. It is expressed extensively in the cytosol of the outer segments of rod and cone photoreceptors, and also in bipolar cells. It is a calcium-dependent protein with a primary function of regulation and inhibition of rhodopsin kinase; an enzyme involved in the rhodopsin phosphorylation process during light and dark adaptation. By inhibiting rhodopsin kinase, recoverin allows for prolongation of the phototransduction cascade and ultimately, light sensitivity [9].
Recoverin is encoded by the RCVRN gene, and is found in the short arm of chromosome 17, localised in the region p13.1 [10]. This is a region populated with cancer-related loci, including the p53 tumour suppressor gene. In the pre-malignant phase, a cancer cell undergoing tumourogenesis can experience a single mutation that inactivates the p53 gene. This can initiate the expression of various other genes, including the RCVRN gene, and can lead to novel production and expression of recoverin by the neoplasm. Recoverin is widely expressed in various different cancers. These include small cell lung cancers, endometrial carcinomas, uterine sarcomas and cervical cancers [7,10,11].
Aberrant expression of recoverin by cancer cells is highly immunogenic. As a consequence of the anti-tumour response by the immune system, recoverin antigens are released into the microenvironment, and are processed by antigen-presenting cells. This can induce the humoral immune response and production of antibodies specific for recoverin antigens. This can prove to be extremely beneficial in the initial anti-cancer response, and can significantly halt the further growth of the neoplasm [7,10,11].
Under normal physiological conditions, the microenvironment of the eye is protected from immunological surveillance by the blood-retinal barrier. This provides retinal protection against autoantibodies generated by a systemic humoral response. It is thought that cancer cells can produce vascular endothelial growth factor (VEGF), which can bind to VEGF receptors located within the retinal vasculature. This can lead to loss of pericytes within this vasculature, resulting in increased permeability of the blood-retinal barrier. As a consequence, the immune privileged status of the eye is lost, and anti-recoverin antibodies can enter the retina.
Cytotoxic T lymphocytes specific to recoverin have also been discovered in blood samples of patients with CAR, and it is proposed that the cellular immune system can also play a significant contribution towards photoreceptor death. Cytotoxic T-lymphocyte antigen 4 (CTLA-4) is a receptor located on activated and regulatory T cells. Once activated, it inhibits interleukin 2, thus playing a crucial role in down-regulating the T-lymphocyte response. Antagonism of CTLA-4 leads to continuous activation of T cells, and further retinal damage. It appears that CTLA-4 blocking is necessary for CAR progression, and explains why many patients with anti-recoverin antibodies do not develop the disease [10].
Another antigen implicated in the pathogenesis of CAR is alpha-enolase. This is a universally expressed 46-kDa glycolytic enzyme, that can be found on the cell membrane surfaces and within the cytoplasm of rod and cone photoreceptors, retinal ganglion cells and muller cells [12]. It is crucially involved in the glycolysis pathway, and converts 2-phosphoglycerate into phosphoenolpyruvate, which produces adenosine triphosphate (ATP). Alpha-enolase is also expressed in many cancer cells and can be released during tumour cell proliferation. Anti-alpha enolase retinopathy has been implicated in cases of lung, breast, prostate, bladder and gastroinestinal tract carcinomas [12].
Antibodies produced against alpha-enolase disrupts the metabolic glycolysis pathway, ATP production, and consequentially induces apoptosis via intracellular calcium ion influx. It has also been shown to reduce intracellular pH, further augmenting the destructive process. All these changes contribute to significant retinal degeneration and visual impairment over a gradual period of time [12].
Altogether, over 18 different auto-antibodies have been identified that have contributed to the pathogenesis of CAR. Out of these, the anti-recoverin antibody has been the most extensively studied and researched (Table 2).

Diagnosis
CAR can take many months to manifest, and diagnosis is challenging and often delayed. Furthermore, there is significant heterogeneity in clinical phenotypes, with presentation of visual disturbance varying greatly. This challenge in diagnosis is compounded further by a commonly normal fundus examination.
Despite this, some general clinical observations can be made. CAR is a bilateral, often asymmetric retinopathy, that can usually precede the diagnosis of malignancy by up to seven months [13]. The presenting complaint is commonly a gradual decline in visual function, which can progress to complete vision loss over a period of days to years. Since common antigen targets are present in all photoreceptor cells, both rods and cones tend to be affected equally in anti-recoverin CAR. Loss of cone photoreceptors causes symptoms of photosensitivity, loss of colour vision, worsening visual acuity, central scotomas, glare and flickering lights. Rod destruction leads to night blindness, poor dark adaptation, peripheral visual field defects and ring scotomas.
On examination, the fundus can appear to be normal. In some patients, attenuation of arterial vasculature can be observed, which can be attributed to retinal vasculitis (Table 1). Optic disc pallor and chorioretinal or retinal pigment epithelium atrophy are also common features. In a small select group of patients, pan-uveitis, vitritis and iritis have also been described [14].
The initial focus in patients with symptoms and signs of autoimmune retinopathy should be to exclude systemic malignancy. Chest x-rays are a useful tool in detecting the presence of any round, cavitating lesions that may suggest adeno-squamous carcinoma of the lung. CT scans of chest, abdomen and pelvis have good utility in identifying occult neoplasms in patients with sinister features of weight loss, night sweats, loss of appetite and change in bowel habit.


Figure 1: Visual fields are markedly diminished, R>L.
Kinetic visual field testing (Figure 1) can be used to detect central, ring or arcuate scotomas, and may be superior to static perimetry. It can also help to identify enlargement of the blind spot and peripheral field defects. Ocular coherence tomography (OCT) is imperative to identify macular oedema, loss of reflectivity and disruption of the photosensitive layers, and may guide further specialised investigations (Figure 2) [15]. Choroidal neovascularisation is an extremely rare complication of CAR, but should be investigated with fluorescein angiography to demonstrate presence of leaky blood vessels, specifically around the optic nerve head [14].
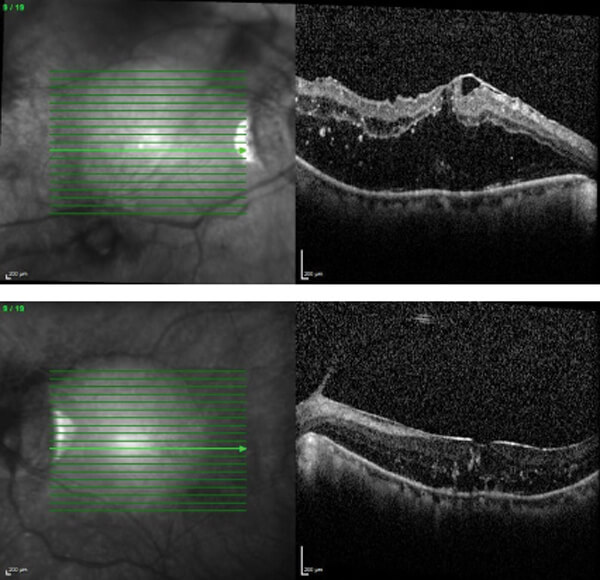
Figure 2: OCT scan showing bilateral macular oedema.
All patients with suspected CAR should undergo electrophysiological studies, as they prove to be valuable diagnostic aids. The different neural elements that contribute to the optical pathways all generate electrical activity, which can be measured to provide crucial information about the integrity of these neural cells [16]. Different electrophysiological patterns help to determine different pathologies in the various layers and cells of the retina. Furthermore, an association between clinical phenotype in CAR and electrophysiological data can be observed in many cases.
A full-field ERG provides information about activity in cones and rod photoreceptor cells, as well as other neural elements. The topographical location of disease within the retina can be determined by multifocal ERG studies. The electrooculogram (EOG) provides information about the integrity of the retinal pigment epithelium (RPE). The ‘a’ wave response to a bright flash of light exclusively details photoreceptor function. The b wave represents activity in the different retinal cells, including bipolar cells. Oscillatory potentials (OP) reflects activity in a complex intersystem between bipolar, amacrine and interplexiform cells [16].

Figure 3: ERG shows very flattened rod and cone profiles.
In CAR, the typical electrophysiological studies will reveal diminished a and b wave amplitudes, with occasionally flattened full-field ERG profiles (Figure 3). In some patients, reduced oscillatory potentials can be observed. In anti-recoverin CAR, there is a homogenous involvement of rod and cone photoreceptors, showing reduced amplitudes with normal or prolonged implicit times. In anti-alpha enolase CAR, there is predominantly more significant global cone damage. Electronegative ERGs can also be observed in some cases [17].
Antibody testing to detect potential contributory autoantibodies in the pathogenesis of CAR is an evolving practice that provides improved diagnosis, classification and prognostication [18]. It generally involves various techniques to determine autoantibodies to components of retina, including immunohistochemistry, western blotting, enzyme-linked immunoassay (ELISA), cytotoxicity assays and multiplex assays. Different tests can be assimilated to provide detailed immunological information.
Unfortunately, only one specific autoantibody is being investigated during each cycle of testing, and with the identification of numerous antigen targets, extensive testing in various different laboratories would be required. Furthermore, there seems to be a lack of methodological standardisation amongst different institutes, which can potentially provide different experimental results. In addition, anti-retinal antibody titres may vary significantly during the clinical course, which could affect interpretation of serial results. Finally, there is an unknown predictive value of a positive test [17].
Management
Visual prognosis of untreated CAR is poor. Many patients will progress to severe visual impairment, with minimal light perception. Therefore, early intervention is critical. Unfortunately, there seems to be no standardised, evidence-based approach to manage and reduce disease severity in CAR. Removal of the primary tumour and cancer regression can cause a decline in circulating autoantibodies, however it does not influence CAR progression [11].
Treatment is based on the idea that CAR is an autoimmune condition, and achieving good immunological control via various immunomodulatory agents is key. Oral corticosteroids have been used frequently in a number of studies, with antibody titre being the principle measured outcome. Results have been largely inconsistent, however there seems to be positive results when used short-term in anti-recoverin CAR. It has been postulated that if one immunosuppressive agent is ineffective, an alternative should be tried. Intravenous methylprednisolone can provide significant short-term visual improvement when coupled with cancer removal.
Cyclosporin, azathioprine and mycophenolate mofetil have also been tried unsuccessfully due to significant drop out rates as a result of undesirable severe systemic side- effects. Intravenous immunoglobulins and plasmaphoresis have also been implemented, often in combination with corticosteroids, with some cases of visual improvement. Failing this, patients can be tried on newer monoclonal antibodies, including alemtuzumab (a CD52 pan-lymphocytic antibody) and rituximab (CD20 B cell antibody). Results of these newer biologics have been promising [19,20].
The focus of future research lies in the use of calcium channel blockers to prevent intracellular calcium ion influx into photoreceptors, thus preventing the caspase induced apoptotic pathway. Gene transfer of ciliary neurotrophic factor is a novel procedure that can also play a crucial role in arresting cellular apoptosis of photoreceptor cells. Anti-vascular endothelial growth factor (VEGF) may help prevent antibodies permeating the retinal-blood barrier. So far, these novel therapies have been only used in animal models, and human studies are required to assess safety and efficacy [11].
Currently, there seems to be profound limitations in producing an evidence-based standardised model in the management of CAR. This is because sample sizes in existing studies have been extremely small, involving only a few patients. Furthermore, participating volunteers have displayed significant heterogeneity in clinical and immunological characteristics. Diagnosis is also very much delayed, and only ascertained after intensive investigation and exclusion of other possible causes. By this time, the degree of visual impairment is permanent and prognosis is poor. Ideally, a prospective, multicentre, double-masked, randomised control trial would inform future clinical practice [7].
References
1. Bussat A, Langner-Lemercier S, Salmon A, Mouriaux F. Paraneoplastic syndromes in ophthalmology. J Fr Ophthalmol 2018;41:e181-e5.
2. Sawyer RA, Selhorst JB, Zimmerman LE, Hoyt WF. Blindness caused by photoreceptor degeneration as a remote effect of cancer. Am J Ophthalmol 1976;81:606-13.
3. Kornguth SE, Klein R, Appen R, Choate J. Occurrence of anti‐retinal ganglion cell antibodies in patients with small cell carcinoma of the lung. Cancer 1982;50:1289-93.
4. Thirkill CE, Tait RC, Tyler NK, et al. The cancer-associated retinopathy antigen is a recoverin-like protein. Investig Ophthalmol Vis Sci 1992;33:2768-72.
5. Elsheikh S, Gurney SP, Burdon MA. Melanoma‐associated retinopathy. Clin Exp Dermatol 2020;45:147-52.
6. Gass JDM, Gieser RG, Wilkinson CP, et al. Bilateral diffuse uveal melanocytic proliferation in patients with occult carcinoma. Arch Ophthalmol 1990;108;527-33.
7. Braithwaite T, Vugler A, Tufail A. Autoimmune retinopathy. Ophthalmologica 2012;228:131-42.
8. Foster S, Vitale A: Diagnosis & Treatment of Uveitis. Jaypee Highlights Medical Publishers Inc: Panama City, Panama; 2013.
9. Mannu GS. Retinal phototransduction. Neurosciences 2014;19:275-80.
10. Adamus G. Are anti-retinal autoantibodies a cause or a consequence of retinal degeneration in autoimmune retinopathies? Frontiers in Immunology 2018;9:1.
11. Shildkrot Y, Sobrin L, Gragoudas ES. Cancer-associated retinopathy: update on pathogenesis and therapy. Semin Ophthalmol 2011;26:321-8.
12. Adamus G, Amundson D, Seigel GM, Machnicki M. Anti-enolase-α autoantibodies in cancer-associated retinopathy: Epitope mapping and cytotoxicity on retinal cells. J Autoimmun 1998;11:671-7.
13. Thirkill CE, Keltner JL, Tyler NK, Roth AM. Antibody reactions with retina and cancer-associated antigens in 10 patients with cancer-associated retinopathy. Arch Ophthalmol 1993;111:931-7.
14. Weleber RG, Watzke RC, Shults WT, et al. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am J Ophthalmol 2005;139:780-94.
15. Abazari A, Allam SS, Adamus G, Ghazi NG. Optical coherence tomography findings in autoimmune retinopathy. Am J Ophthalmol 2012;153:750-6.
16. Holopigian K, Hood DC. Electrophysiology. Ophthalmol Clin North Am 2003;16:237-51.
17. Link B, Schlötzer-Schrehardt U, Jünemann A. Carcinoma-associated retinopathy-an electrophysiological and immunohistochemical correlation. Retina 2009;29:6972.
18. Adamus G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmunity Reviews 2009;8:410-4.
19. Heckenlively JR, Ferreyra HA. Autoimmune retinopathy: A review and summary. Semin Immunopathol 2008;30:127-34.
20. Guy J, Aptsiauri N. Treatment of paraneoplastic visual loss with intravenous immunoglobulin: Report of 3 cases. Arch Ophthalmol 1999;117:471-7.
TAKE HOME MESSAGE
-
CAR is caused by tumour cells expressing retinal photoreceptor antigens which elicits a systemic immune response that can cross-react with the retina.
-
Symptoms reflect involvement of both rod and cone photoreceptors, and include severely reduced visual acuity, photopsia, colour impairment, photosensitivity, central or peripheral ring scotomas, nyctalopia.
-
Fundus examination is often unremarkable, but may demonstrate optic nerve pallor, attenuated retinal vasculature, vitreous cells, RPE changes.
-
ERG findings are illustrative of global retinal dysfunction, with severely flattened scotopic and photopic a and b waves.
-
Corticosteroids, plasmaphoresis and IV immunoglobulin can be used in combination, often with poor outcomes.
-
Overall, the visual prognosis is poor, commonly resulting in vision loss.
COMMENTS ARE WELCOME






