Presentation
A 46-year-old Caucasian female was referred to the eye clinic by her local optician following a routine sight test. She was noted to have pigmentary retinal changes in both eyes but was asymptomatic with no visual complaints. At presentation she described no difficulties with night vision, no history of symptoms suggestive of past intraocular inflammation and was unaware of any visual field difficulties.
The patient had previously been seen elsewhere 13 years ago with regards to these findings. The referring optician was concerned that there had been some progressive changes in the right eye since this time.
Background
The patient had no other past ocular history, no significant past medical history and was otherwise fit and well and on no regular medications. There was also no relevant family history. The patient drove and was a current smoker.
Examination
On examination, her best corrected visual acuity was 6/4 in the right eye and 6/5 in the left eye. Intraocular pressures were 14mmHg in the right eye and 16mmHg in the left eye. Her refraction was +1.00/-4.25 x 18 in the right eye and +0.50/-4.25 x 153 in the left eye. The anterior segment examination with unremarkable with white, quiet eyes, clear corneas, quiet anterior chambers and clear crystalline lenes. There were no anterior vitreous cells and a clear vitreous with no haze, snowballs or snowbanking.
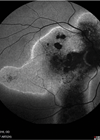
Fundus examination was quite striking in both eyes with bilateral, symmetrical findings. There was evidence of pigment accumulation along the retinal veins, chorioretinal atrophy distributed along the retinal vasculature and elevated, blurred disc margins with a ‘lumpy’ appearance in both eyes.


Figures 1a and 1b: Widefield fundus imaging demonstrating pigment accumulation along the retinal veins (white arrows), chorioretinal atrophy (red arrows) and disc drusen (green arrows).
Investigation
Widefield fundus imaging demonstrated these clinical findings (Figures 1a, 1b). Fundus autofluorescence showed hypo- autofluorescence in the areas of pigment accumulation and hyperautofluorescence in the areas of chorioretinal atrophy (Figures 2a, 2b). Fundus autofluorescence also showed hyper-autofluorescence at the optic disc indicative of optic nerve drusen. A 30-2 Humphrey visual field test revealed some defects superiorly paracentrally in the right eye. In the left eye there was a more significant field defect with an inferior and superior arcuate defect noted.


Figures 2a and 2b: Widefield fundus autofluorescence demonstrating hypo-autofluorescence in areas of pigment accumulation (white arrows), hyper-autofluorescence in areas of chorioretinal atrophy (red arrows) and hyper-autofluorescent optic nerve head drusen (green arrows).
Diagnosis
The characteristic fundus findings were in keeping with pigmented paravenous retinochoroidal atrophy (PPRCA). The secondary diagnosis of optic disc drusen was felt to be incidental and unrelated.
Further investigation
The patient was enrolled in the 100,000 genomes project but this did not identify any underlying genetic cause for her clinical presentation. The patient also had bloods to exclude a treatable, infective cause for the fundus findings. Treponema serology and tuberculosis T-Spot was negative.

Figure 3: OCT through the retinal vasculature demonstrating thinning
of the outer retina and loss of the ellipsoid zone (white arrow).
Follow-up
The notes from her previous ophthalmology assessment 13 years previously were requested and reviewed. These notes revealed that a diagnosis of bilateral perivascular pigmentary dystrophy and disc drusen had previously been made. At this point she had some patchy peripheral field loss in both eyes although the reliability of the test was questioned. The patient had electrodiagnostic tests after this initial presentation which showed a reduced a/b ratio on standard flash electroretinogram, borderline amplitude scotopic responses and abnormally reduced photopic responses. Pattern electroretinogram was normal and electrooculogram showed a reduced Arden index.
The clinical notes and diagrams from these initial consultations suggested that the fundus appearances and visual field defects were bilateral and not significantly progressive over the past 13 years.


Figures 4a and 4b: Macula OCT demonstrating normal anatomy at the central macula and fovea.
The patient was followed up annually for a period of monitoring over three years. At follow-up consultations and with deeper questioning, the patient did report some mild difficulty with night vision and symptoms of glare. However, she had no significant visual complaints and there was no functional impact on her quality of life.
Her fundus appearances remained largely unchanged over three years aside from a slight increase in pigment deposition within the same distribution. Her visual acuity remained excellent at 6/6 or better in both eyes at each visit. The visual field remained stable or only very slowly progressive. Macula OCT at each visit remained stable and dry.
Discussion
Introduction and terminology
Pigmented paravenous retinochoroidal atrophy is a rare form of chorioretinal atrophy of unknown aetiology. It is characterised by retinal pigment epithelium degeneration, choriocapillaris atrophy and perivenous pigment deposition [1]. Its first description was provided by T Hewitson Brown in 1937 in a 47-year-old Danish man with a previous tuberculosis exposure whose fundoscopic findings were remarkable for chorioretinal atrophy along the retinal veins, described as “beads on a string” with associated pigment clumps. Then, the condition was termed ‘retino-choroiditis radiata’ and tuberculosis was postulated to be an association [2]. Since then, this condition has been referred to by various appellations including pseudoretinitis pigmentosa following measles, chorioretinitis striata, congenital pigmentation of the retina, melanosis of the retina, paravenous chorioretinal degeneration, pigmented paravenous chorioretinal atrophy (PPCRA) and PPRCA. The name PPRCA is perhaps preferred over PPCRA as it is generally agreed that RPE is the primary site of involvement, with secondary atrophy of the underlying choroidal vasculature [3].
Aetiology
The majority of PPRCA cases appeared to be sporadic. In the literature, cases of familial occurrence have also been described. Nevertheless, a specific mode of transmission has not been proven. At present, only a handful of genetic mutations have been hypothesised to be associated with PPRCA such as the c.619G->A heterozygous mutation in the CRB1 gene as reported by McKay, et al. in a family with dominantly inherited PPCRA [4].
A degenerative nature of the condition was also proposed, such as in a 1992 case report by Chen M-S, et al. which described a patient with bilateral macular coloboma and PPRCA thought to be sequelae of developmental abnormality [5].
Inflammatory and infectious aetiologies have also been proposed with cases reporting an association between PPRCA and conditions such as Behçet’s disease [6], syphilis [7], measles [8], rubeola [9] and Vogt-Koyanagi-Harada disease [10]. However, it is currently agreed that these inflammatory retinal vein vasculopathies, whilst may present with similar phenotypes, should be termed ‘pseudo-PPRCA’ [3].
Pathology
To date, there is no known histopathological study on a human eye with PPRCA. Linek, et al. described five cases of PPRCA in dogs and performed a histological examination in one. The authors described severe multifocal perivascular retinal atrophy characterised by loss of photoreceptor outer segments, outer nuclear layer and inner nuclear layer. The inner plexiform layer and ganglion cell layer (GCL) were also reduced [11].
In human eyes, descriptions of histopathological change are based on multimodal imaging techniques including spectral-domain-OCT (SD-OCT) and fluorescein angiography (FFA), as well as visual electrophysiology. In a retrospective study by Lee, et al., SD-OCT imaging of retinochoroidal atrophic areas in PPRCA showed thinning of the outer nuclear layer (ONL), external limiting membrane (ELM), ellipsoid zone (EZ) and interdigitation zone (IZ) band. Reduced choroidal thickness is another feature even in the absence of RPE atrophy. Pigmented paravenous retinochoroidal atrophy also appears to primarily affect choroidal vasculature as evidenced by findings in keeping with choriocapillaris hypoperfusion on OCT angiography, with relative sparing of the retinal capillary plexuses [12]. Macula may be involved and this is characterised by disruptions of the EZ, IZ, and RPE, as well as choroidal thinning [10,12].
Signs and symptoms
The presentation of PPRCA is variable and patients are often asymptomatic with the finding of PPRCA being incidental. Symptoms may manifest as mild blurred vision, nyctalopia and reduced peripheral vision [12]. Significantly reduced acuity was also described in cases with macular involvement [12,13]. Nyctalopia is uncommon in PPRCA and warrants consideration of other conditions such as retinitis pigmentosa (RP) [3].
Pigmented paravenous retinochoroidal atrophy is characterised by bilateral paravenous retinochoroidal atrophy and pigment accumulation. Pigmentation may be fine, coarse clumping or bone corpuscle pigmentation [14]. However, the phenotype can be variable and the distribution of pigmentary changes has been used to classify PPRCA. The pigmentary changes in PPRCA can be ‘paravenous’, ‘focal’, or ‘confluent’ where extensive retinochoroidal atrophy with bone spicules merges with one another, mimicking RP [12]. Pigmented paravenous retinochoroidal atrophy can also be described in terms of severity ranging from mild, intermediate to severe. In the mild form, there are only a few scattered areas of minimal retinochoroidal atrophy and paravenous pigmentation. Paravenous pigmentation around the majority of retinal veins associated with minimal or regional retinochoroidal atrophy is seen in intermediate cases. Severe cases are characterised by diffuse retinochoroidal atrophy, with extensive and heavy paravenous pigment accumulation [3]. Atrophic area may spread into the periphery in a fin-like appearance [14]. Choroidal vessels may be visible in areas with severe retinochoroidal atrophy [14].
Other unusual ocular findings have been reported including cystoid macular oedema, epiretinal membrane, lamellar or full-thickness macular holes, nystagmus and eso- and exotropia [1].
Diagnostic imaging
Fundus autofluorescence
Fundus autofluorescence shows hypo-autofluorescence in areas of RPE atrophy, surrounded by hyper-autofluorescence due to lipofuscin accumulation in areas of RPE dysfunction [16].
Optical coherence tomography
Optical coherence tomography findings include absent EZ and ELM, thinning of ONL and outer plexiform layer, partial preservation of RPE, and choroidal thinning. Retinal nerve fibre layer and GCL can also be thin, and in cases with macular atrophy, thinning of all retinal layers may be present [17]. Pigment clumps appear as hyper-reflective plaques with underlying shadowing [15]. Optical coherence tomography angiography findings are characterised by vascular impairment, especially at the level of deep capillary plexus [17].
Fluorescein angiography & indocyanine angiography (ICG)
Hyper-fluorescence due to window defects from RPE atrophy can be seen on FFA. Areas of pigment clumping will correspond to hypofluorescence due to blockage effect [3]. Hypo-fluorescent areas on ICG indicating choriocapillaris atrophy are more extensive than those on FFA [18].
Electroretinogram & electro-oculogram
Electroretinogram and electro-oculogram findings can vary significantly reflecting the heterogeneity of the condition with different involvement of different cell types of the retina and RPE [3,18].
Management
Pigmented paravenous retinochoroidal atrophy is a non-progressive or slowly progression condition with no treatment required.
Prognosis
Pigmented paravenous retinochoroidal atrophy is a non-progressive or slowly progressive disease with a good visual prognosis, except in cases with macular involvement. Progressive cases may be associated with more severe fundal changes and may not represent true PPRCA [3].
References
1. Antropoli A, Arrigo A, Pili L, et al. Pigmented paravenous chorioretinal atrophy: Updated scenario. Eur J Ophthalmol 2023;34(4):941–51.
2. Brown TH. Retino-choroiditis radiata. Br J Ophthalmol 1937;21:645–8.
3. Huang HB, Zhang YX. Pigmented paravenous retinochoroidal atrophy (Review). Exp Ther Med 2014;7(6):1439–45.
4. McKay GJ, Clarke S, Davis JA, et al. Pigmented paravenous chorioretinal atrophy is associated with a mutation within the crumbs homolog 1 (CRB1) gene. Invest Ophthalmol Vis Sci 2005;46:322–8.
5. Chen MS, Yang CH, Huang JS. Bilateral macular coloboma and pigmented paravenous retinochoroidal atrophy. Br J Ophthalmol 1992;76(4):250–1.
6. Kellner U, Helbig H, Foerster MH. Phänokopien hereditärer Netzhautdegenerationen [Phenocopies of hereditary retinal degenerations]. Ophthalmologe 1996;93(6):680–7.
7. Chi HH. Retinochoroiditis radiata. Am J Ophthalmol 1948;31(11):1485–7.
8. Peduzzi M, Guerrieri F, Torlai F, Prampolini ML. Bilateral pigmented paravenous retino-choroidal degeneration following measles. Int Ophthalmol 1984;7(1):11–4.
9. Foxman SG, Heckenlively JR, Sinclair SH. Rubeola retinopathy and pigmented paravenous retinochoroidal atrophy. Am J Ophthalmol 1985;99(5):605–6.
10. Ramtohul P, Comet A, Gascon P, et al. Pigmented paravenous retinochoroidal atrophy associated with Vogt-Koyanagi-Harada disease: a case report. BMC Ophthalmol 2020;20(1):36.
11. Linek J, Gruber AD, Mecklenburg L. Five cases of pigmented paravenous retinochoroidal atrophy in dogs. Vet Ophthalmol 2012;15Suppl2:41–7.
12. Lee EK, Lee SY, Oh BL, et al. Pigmented Paravenous Chorioretinal Atrophy: Clinical Spectrum and Multimodal Imaging Characteristics. Am J Ophthalmol 2021;224:120–32.
13. Murray AT, Kirkby GR. Pigmented paravenous retinochoroidal atrophy: a literature review supported by a unique case and insight. Eye (Lond) 2000;14 Pt 5:711–6.
14. Takagi S, Hirami Y, Takahashi M, et al. Use of Wide-Field Fundus Camera, Fundus Autofluorescence, and OCT in Cases of Pigmented Paravenous Retinochoroidal Atrophy. Ophthalmol Retina 2018;2(1):79–81.
15. Ghosh B, Goel N, Batta S, Raina UK. SD-OCT in pigmented paravenous retinochoroidal atrophy. Ophthalmic Surg Lasers Imaging 2012;43(3):e41–3.
16. Hashimoto Y, Kase S, Saito W, Ishida S. Abnormalities of fundus autofluorescence in pigmented paravenous chorioretinal atrophy. Open Ophthalmol J 2012;6:125–8.
17. Battaglia Parodi M, Arrigo A, Chowers I, et al. OPTICAL COHERENCE TOMOGRAPHY ANGIOGRAPHY FINDINGS IN PIGMENTED PARAVENOUS CHORIORETINAL ATROPHY. Retina 2022;42(5):915–22.
18. Yanagi Y, Okajima O, Mori M. Indocyanine green angiography in pigmented paravenous retinochoroidal atrophy. Acta Ophthalmol Scand 2003;81(1):60–7.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME