Intravitreal dexamethasone (Ozurdex 0.7mg) is a biodegradable, sustained-release implant used to manage diabetic macular oedema, macular oedema secondary to retinal vein occlusion, and non-infectious posterior uveitis due to its anti-inflammatory effects and VEGF suppression [1].
While effective, the implant may cause side-effects such as elevated intraocular pressure (IOP) and cataracts. Rarely, it migrates into the anterior chamber (AC), risking corneal oedema and decompensation [2]. Khor, et al. reported 81.9% of such cases developed corneal oedema and 31.4% progressed to decompensation despite treatment [3].
Corneal damage may result from the implant’s composition, dexamethasone, glycolic acid, lactic acid, or mechanical trauma [4]. Prolonged contact with the endothelium may trigger apoptosis and cell loss. Therefore, prompt removal is critical. This report outlines a minimally invasive technique using a bevelled 18G intravenous (IV) cannula for implant removal, with a review of alternative surgical approaches.
Case report
A 70-year-old man presented with severe pain and blurred vision in his right eye (RE) four days after intravitreal dexamethasone administration. His history included childhood trauma causing zonular instability, followed by multiple lens exchanges and eventual Yamane scleral-fixated intraocular lens (IOL) placement. This resulted in persistent cystoid macular oedema which had been treated with the dexamethasone implant.
His visual acuity was 1/60 RE and 6/6–2 left eye (LE). Slitlamp examination showed corneal decompensation and a freely floating dexamethasone implant in the AC. Intraocular pressure was 8mmHg RE and 15mmHg LE. Immediate surgical removal was planned.

Figure 1: A 2.2mm temporal corneal incision is made.
Surgical technique
The dexamethasone implant was removed in the operating theatre under sub-tenon anaesthesia. A 2.2mm temporal corneal incision was made (Figure 1). Intracameral pilocarpine (5%) was administered to constrict the pupil, and a cohesive ophthalmic viscoelastic device (OVD) was introduced to aid in mobilising the implant within the AC to align with the corneal incision (Figure 2).

Figure 2: Introduction of OVD and mobilisation of the implant.
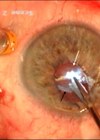
The implant was then ensheathed by an 18G intravenous (Venflon) cannula and successfully aspirated (Figure 3).

Figure 3: Attempting to secure and aspirate the implant using an 18G intravenous cannula.
The Venflon cannula had been modified by shortening and bevelling the tip to improve the manipulation of the cannula within the AC. Bevelling the cannula facilitated capture of the implant within the scaffold of the cannula. The cannula had been attached to a 1ml syringe to provide fine control of aspiration of the implant. The OVD was removed using a Simcoe cannula, the corneal wound was hydrated and the patient received intracameral cefuroxime and subconjunctival dexamethasone at the end of the procedure.
Postoperative outcome and follow-up
The patient was prescribed dexamethasone, neomycin and polymyxin B (Maxitrol) eye drops to be administered twice daily for two weeks, along with acetazolamide 250mg tablets, dosed at twice daily for three days. Three days later, RE visual acuity improved slightly to 3/60, and corneal oedema reduced, but Descemet’s membrane folds and iris atrophy were noted. As the implant was removed, further steroid use was contraindicated.
Acetazolamide tablets were discontinued, and the patient was prescribed sodium chloride 5% eye drops twice daily for the RE to address his corneal oedema. At three months, RE vision remained at 3/60 with persistent corneal decompensation and a corneal graft was advised.
Discussion
Anterior migration of dexamethasone implant is a sight-threatening complication that should be considered in high-risk individuals. A case series by Kayikcioglu, et al. identified key risk factors for implant migration, including prior vitrectomy, complicated cataract surgeries and retinal detachment surgeries with sutured IOLs [5]. In our case, the patient had undergone a vitrectomy with peripheral iridotomy enabling communication between the anterior and posterior chamber. Identifying these risk factors is crucial in informing management decisions on the use of intravitreal dexamethasone and prompt identification of complications.
The use of a manually bevelled intravenous cannula with fine aspiration is a simple, cost-effective method for removal. This technique, as demonstrated in our case, offers minimal implant manipulation, reducing the risk of implant fracture. Tissue manipulation is also minimal as it is compatible with smaller corneal incisions, which are particularly advantageous in preserving the integrity of the cornea. Furthermore, IV cannulas are cost-effective, widely available and easily adaptable to various clinical settings, making this method an appealing option when specialised ophthalmic instruments are unavailable. The simplicity and safety of this technique are its main advantages, particularly in emergency or resource-limited environments.
Supporting the cannula technique, Ku, et al. reported a case, successfully utilising the IV cannula aspiration technique with a 16G Venflon for the removal of a migrated dexamethasone implant [6]. In contrast, our approach involved a manually bevelled 18G cannula, which provided enhanced control during the aspiration process. The bevelled design facilitated precise manipulation of the implant, while the smaller gauge allowed for a reduced corneal incision size, minimising risks of wound leakage, anterior chamber shallowing and hypotony. Notably, the Ozurdex implant has a diameter of approximately 0.46mm [7], compared to the internal diameter of 16G (1.65mm) and 18G (1.27mm) cannulas. This suggests that using a smaller cannula, such as our 18G modification, aligns closely with the implant’s dimensions, ensuring secure aspiration while preserving corneal integrity. These refinements highlight the adaptability of the IV cannula technique and its potential to reduce surgical trauma compared to larger gauge cannulas. Nonetheless, like Ku, et al., our patient required a corneal graft, underscoring the importance of addressing pre-existing ocular damage associated with anterior migration.
Alternatively, forceps can be used to extract a dexamethasone implant from the AC. While forceps provide precision and control during the procedure, they come with notable risks. A case series by Kang, et al. highlighted that forceps use can lead to corneal endothelial damage, an undesirable outcome given the fragile nature of corneal endothelium [8]. Moreover, direct manipulation of the implant carries a higher risk of implant fracture. This risk of iatrogenic trauma suggests that while forceps may offer a more controlled method of implant removal, the potential for further corneal injury makes the IV cannula technique a safer choice in such cases.
Another notable technique is the ‘no-touch’ method, which minimises potential trauma to the corneal endothelium [9].
This approach, like the IV cannula technique, employs a viscoelastic material to manipulate the implant into position without direct contact [9]. By inflating the chamber carefully, the implant can be reorientated and floated toward the corneal incision, allowing for removal in a single piece [9]. However, this technique relies heavily on the precise use of viscoelastic material, which can be difficult to control, especially if the implant breaks into smaller fragments [9]. Furthermore, it requires a larger corneal wound and leaking the implant through the corneal incision may be less controlled than fine direct aspiration of it. In such cases, incomplete removal can occur, which may require additional interventions to ensure all implant components are cleared from the AC.
In milder cases without decompensation, repositioning may suffice. Ahuja, et al. used a 30G needle to reposition the implant into the vitreous with good outcomes [10]. However, this is less suitable in cases with existing corneal damage or high recurrence risk.
Conclusion
A bevelled 18G IV cannula provides a simple, safe and effective approach for removing migrated dexamethasone implants from the AC. Its low cost and minimal invasiveness make it ideal in both emergency and resource-limited settings. However, treatment must be tailored to individual clinical circumstances. In selected cases, repositioning may suffice, but timely surgical removal remains critical to avoid irreversible corneal damage.
References
1. Augustin AJ, Kuppermann BD, Lanzetta P, et al. Dexamethasone intravitreal implant in previously treated patients with diabetic macular edema: subgroup analysis of the MEAD study. BMC Ophthalmol 2015;15:150.
2. Röck D, Bartz-Schmidt KU, Röck T. Risk factors for and management of anterior chamber intravitreal dexamethasone implant migration. BMC Ophthalmol 2019;19:120.
3. Khor HG, Lott PW, Wan Ab Kadir AJ, et al. Review of risk factors and complications of anterior migration of Ozurdex implant: lessons learnt from previous reports. J Ocul Pharmacol Ther 2024;40(6):342–60.
4. Tsoutsanis P, Kapantais D. Anterior migration of Ozurdex implant: a review on risk factors, complications, and management. Int J Retin Vitr 2023;9:74.
5. Kayıkcıoğlu Ö, Doğruya S, Sarıgül C, et al. Anterior chamber migration of Ozurdex implants. Turk J Ophthalmol 2020;50(2):115–22.
6. Ku JY, Mercieca K, Yau K. Removal of a migrated dexamethasone implant (Ozurdex) from the anterior chamber using an intravenous cannula. BMJ Case Rep 2021;14(7):e240504.
7. https://www.ema.europa.eu/en/documents/
product-information/ozurdex-epar-
product-information_en.pdf
8. Kang H, Lee MW, Byeon SH, et al. The clinical outcomes of surgical management of anterior chamber migration of a dexamethasone implant (Ozurdex®). Graefes Arch Clin Exp Ophthalmol 2017;255:1819–25.
9. Pitcher JD. Techniques for managing anterior migration of Ozurdex implants. Retina Today 2014:
https://assets.bmctoday.net/retinatoday/
pdfs/0714RT_Pearls_Pitcher.pdf
10. Ahuja AS, Jaraki JA, Halperin LS. Office management of Ozurdex implant dislocation into the anterior chamber. Retin Cases Brief Rep 2023;17(2):170–2.
[All links last accessed May 2025]
Acknowledgement: The authors are grateful to the patient for providing consent to publish this case report.
Declaration of competing interests: None declared.