
Glaukos has announced the launch of its latest glaucoma treatment, iStent infinite®, in the UK market after securing approval under the European Medical Device Regulation (MDR). This new approval process is rigorous and provides ophthalmologists with reassurance regarding the efficacy and safety of the device.
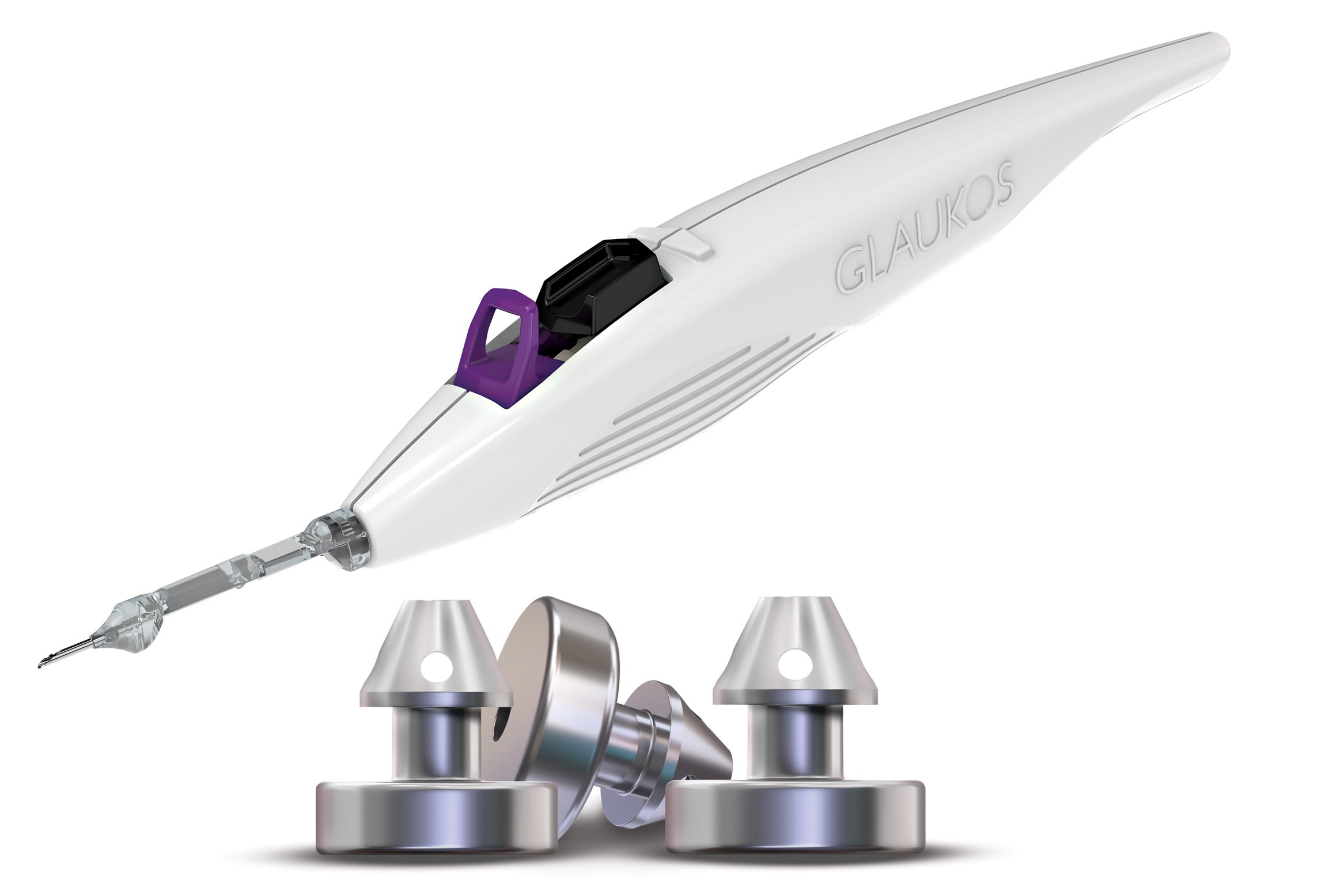
The iStent infinite® builds upon and complements the existing iStent® technologies, which have been proven and trusted in the UK since 2016. With more than one million iStent® devices implanted worldwide, the technology has been shown to delay glaucoma disease progression. iStent infinite® is the only truly microinvasive technology for the treatment of moderate to advanced glaucoma patients, providing powerful IOP reductions and predictable outcomes for those with a high preoperative burden.
Not only does the iStent infinite® offer an additional third iStent®, but its injector system has also been enhanced to allow for unlimited delivery clicks.
The new injector system features include:
- Auto retractor sleeve – prevents viscoelastic egress, improving the view of the angle.
- Offset 8° angle – between the injector and the handpiece, providing a more ergonomic approach and enhanced angulation to the trabecular meshwork (TM).
- Longer viewing slot – increases visualization of the trocar tip during the procedure.
iStent infinite® builds on the extensive iStent® technology dataset, which represents the most robust, diverse, and long-term data of any trabecular micro-bypass (TMB) procedure on the market, and has been proven to be safe and predictable based on pivotal trials.
FURTHER INFORMATION:
For more information on Glaukos and iStent infinite®



