“The beauty of scientific research lies in that the search for answers often yields yet more questions.”
A large body of evidence points to the corneoscleral limbal location as the repository of putative epithelial stem cells [1]. Thoft proposed the X+Y=Z hypothesis to explain the cell dynamics that maintain corneal epithelial homeostasis during health and disease. The loss of corneal epithelial cells (Z) is equal to the sum of the anterior migration of cells from the basal epithelium (X) and the centripetal migration from the limbus (Y) [2].
Stem cells are said to reside within a niche, or environment, which supports their growth and replication and affords them protection. Outside their niche, stem cells would undergo differentiation to form progeny cells named transient amplifying cells (TACs), which have been shown to exhibit highly proliferative properties to heal corneal epithelium in time of injury [1].
How is limbal stem cell deficiency diagnosed?
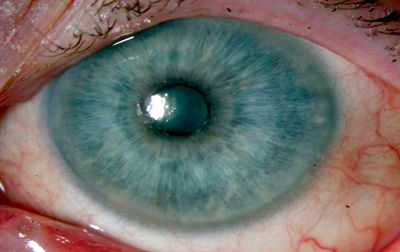
Patients suffering from limbal stem cell deficiency (LSCD) have a decreased ability to restore their population of corneal epithelial stem cells and present with symptoms such as irritation, photophobia and reduced visual acuity. Slit-lamp biomicroscopy findings identify corneal vascularisation, peripheral pannus and late staining of the epithelium with fluoroscein (due to an altered and abnormal corneal epithelial surface). Progression of the condition leads to superficial punctate keratopathy and frank epithelial defects with further scarring, decompensation and, ultimately, perforation (Figure 1). Partial LSCD can involve the visual axis necessitating intervention or can occur excluding the visual axis, when no or minimal intervention with topical medication may be required (Figure 2).
Figure 1a.

Figure 1b.

Figure 1b.
Figure 1: Partial limbal stem cell deficiency sparing visual axis.
1a: Indeterminate features of partial limbal stem cell deficiency with relatively clear visual axis.
1b: Disorganised corneal epithelial surface and healing suture line. Lower arrow indicating edge of conjunctivalisation.
1c: Diffuse fluorescein staining showing poor corneal epithelial healing and marked superficial keratitis.

Figure 2: Partial limbal stem cell deficiency involving visual axis. Conjunctivalisation of superior cornea displaying marked vascularisation with associated scarring and irregular corneal epithelium (negatively stained with fluorescein dye). See arrow.

Figure 3: Total limbal stem cell deficiency following chemical injury. Complete, 360° damage of normal limbal architecture leading to significant conjunctivalisation and loss of corneal transparency. Note marked disruption of central corneal epithelium centrally and symblepharon (see arrows).
How does one manage total limbal stem cell deficiency?
While partial LSCD could, in theory, be managed by observation or debridement of pannus, total LSCD fails to respond to conservative measures (Figure 3). Initial application of conjunctival-limbal transplants showed encouraging results for presumed LSCD [3]. The options of autologous limbal transplants (CLAU) from fellow eyes evolved to allografts from either living relatives (lrCLAL) or cadaveric kerato-limbal allografts (KLALs). DNA analyses with these studies demonstrate positive engraftment (where corneal epithelial cell DNA analysis can be traced back to donor stem cells). However, limitations existed with allografts in that systemic immunosuppression was necessary to reduce the risk of rejection and subsequent graft failure. Furthermore, although autologous grafts did not share this predicament, undiagnosed bilateral LSCD could lead to grafting of damaged limbus, which can both exacerbate the donor eye and lead to failure in the recipient eye. These pitfalls necessitated the need to find superior methods of transplant.
What are ex vivo stem cell transplants?
In recent years, ex vivo expansion of limbal epithelial stem cells has been popular in the treatment of total LSCD [4]. A small amount of donor limbus containing presumed limbal stem cells is expanded ex vivo. This is then transplanted onto the ocular surface, commonly using amniotic membrane as a carrier. These techniques have shown comparable results with conventional limbal sheet transplants [5].
What is the role of the corneoscleral limbus?
The conventional ‘wisdom’ of the limbus as the seat of corneal epithelial regeneration has been in the spotlight in recent years. Interestingly, however, an observational, prospective consecutive case series by Dua et al. seemed to indicate otherwise [6]. Five human subjects (eight eyes) presenting with total LSCD were followed for a mean period of 60 months (range 8-12 years). These patients had confirmed 360 degree LSCD diagnosed via clinical examination and in vivo confocal microscopy (IVCM) showing bright conjunctival epithelial cells, goblet cells and superficial and deep vascularisation. The central cornea, in all cases, contained an island of normal corneal epithelium. To the surprise of the author, this central island persisted throughout the observational period indicating a limited role of the limbal epithelial stem cells in the physiologic homeostasis of corneal epithelium [6]. There appeared to be a collection of cells, in the central cornea, able to self-sustain in the absence of stem cells. The role of transient amplifying cells once again came to the fore. Are these hybrid progeny cells able to retain characteristics of stem cells (i.e. independent regeneration) while also being highly migratory and proliferative? Can this exist outside the established stem cell niche? If not, then how can one reconcile the arguments made in light of the observations seen?
What is the fate of transplanted limbal stem cells?
The fate of transplanted stem cells has produced some interesting findings. While the short and intermediate term results of limbal stem cell transplantation appear promising, evidence for long-term engraftment is conflicting. Techniques using DNA finger-printing have given mixed results on the engraftment of transplanted stem cells. While two studies reported the presence of donor DNA up to three years after limbal graft transplantation, several other reports failed to demonstrate donor cells in the cornea years after grafting [4,7-12]. Interestingly some of the cases that failed to show donor DNA had clinically normal corneal epithelium. The fate of transplanted ex vivo expanded corneal limbal stem cells suffered similarly to limbal tissue transplants. Daya et al. performed a series of ex vivo expanded limbal stem cell transplants to treat corneal stem cell deficiencies arising from underlying causes that included Steven Johnsons Syndrome and severe chemical injury amongst others [13]. They reported a 70% success defined by reduction in vascularity, increased cornea clarity, improvement in visual acuity and the presence of cytokeratin markers showing healthy corneal epithelium. Furthermore, they performed DNA fingerprinting of post-graft corneal epithelium, which failed to detect donor cell survival beyond nine months in spite of normalisation of corneal epithelium.
What has cell therapy shown us?
Cell therapy has been pioneered in other specialties and raises some interesting questions. Plastic surgeons applied cultured autologous dermal epithelial cells to treat massive skin burns and chronic ulcers [14-16]. The initial optimism declined among these surgeons due to inconsistency in the clinical results and several surgeons have questioned the usefulness of cultured epithelial cells [17-20]. Haematologists also had similar experiences with ex vivo expanded haemopoetic stem cells. Hollyok et al. reported that ex vivo expanded haemopoetic cells failed to engraft in subjects with bone marrow failure, but whole marrow transplants successfully engrafted. The authors speculated the normalisation of the corneal epithelium might be due to regeneration of ‘dormant’ host stem cells that are impaired by a pathological insult. Cultured epithelial composites, concurrent treatments with steroids and immune suppression that down regulate the ocular surface inflammation may potentiate this process. This can, consequently, trigger activation of resident inactive stem cells by altering the microenvironment through application of a ‘biological dressing’.
Is conjunctivalisation pathognomonic of LSCD?
Disappointingly, the large body of literature has failed to provide alternative explanations for the constellation of features that are termed ‘limbal stem cell deficiency’. The role of the corneal limbus in epithelial maintenance has been the subject of several publications since Davanger et al. and Cotsarelis et al. advanced the concept of limbal stem cells. Animal experiments that followed demonstrated that destruction of the limbus (the presumed location of limbal stem cells) either by thermal or by chemical injury allowed conjunctiva-derived cells and new blood vessels to migrate onto the corneal surface [21-24]. This led to the speculation that loss of limbal function was responsible for the centripetal conjunctival cell migration across the corneal surface. Consequently, the presence of conjunctiva-derived cells on the corneal surface, clinically termed ‘conjunctivalisation’, became a diagnostic criterion for LSCD and was considered pathognomonic of the condition. Conjunctivalisation can be demonstrated by impression cytology for goblet cells. In the last decade cytokeratin profiles have become available as markers of epithelial origin and have been used as a diagnostic tool.
Could metaplasia simulate LSCD?
The literature on ocular surface disease has provided some alternative explanation for clinical features such as abnormal fluorescein staining, corneal vascularisation and persistent epithelial defects. Metaplasia of the ocular surface epithelium is a well-documented feature, whereby the stratified secretory epithelium is replaced by enlarged non-secretory epithelium and varying degrees of keratinisation. Metaplasia of ocular surface occurs due to chronic irritation and inflammation, such as in severe dry eyes, Stevens-Johnston Syndrome, ocular cicatricial pemphigoid, multiple surgeries and radiation [25-26]. It is interesting to note that Tseng et al. have attributed the persistence of metaplastic changes of the ocular surface epithelium to the development of corneal neovascularisation, ulceration and scarring. We have to note that there is much overlap between the corneal features of metaplasia and clinical features of LSCD, therefore making a diagnosis based on clinical features alone may lead to misdiagnosis. The published literature often does not make a clear distinction between the two conditions.
The presence of goblet cells on the corneal surface has been used to support a diagnosis of LSCD, arguing ‘detection of goblet cells’ by impression cytology analysis as an objective parameter. However, there is clear evidence that all stem cells have been depleted [27]. The origin of goblet cells on the corneal surface requires further scrutiny. Limbal stem cell studies (mainly animal experiments of chemical and thermal ablations) concluded that the absence of the limbus leads to migration of conjunctiva-derived cells including goblet cells onto the cornea surface. However, the possibility of goblet cells arising de novo on the corneal surface (goblet cell metaplasia), due to a change in the corneal micro-environment, has been considered [28].
References
1. Dua HS, Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv Ophthalmol 2000;44(5):415-25.
2. Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci 1983;24(10):1442-3.
3. Dua HS, Gomes JA, Singh A. Corneal epithelial wound healing. Br J Ophthalmol 1994;78(5):401-8.
4. Egarth M, Hellkvist J, Claesson M, et al. Longterm survival of transplanted human corneal epithelial cells and corneal stem cells. Acta Ophthalmol Scand 2005;83(5):456-61.
5. Shortt AJ, Secker GA, Notara MD, et al. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv Ophthalmol 2007;52(5):483-502.
6. Dua HS, Miri A, Alomar T, et al. The role of limbal stem cells in corneal epithelial maintenance: testing the dogma. Ophthalmology 2009;116(5):856-63.
7. Djalilian AR, Mahesh SP, Koch CA, et al. Survival of donor epithelial cells after limbal stem cell transplantation. Invest Ophthalmol Vis Sci 2005;46(3):803-7.
8. Williams KA, Brereton HM, Aggarwal R, et al. Use of DNA polymorphisms and the polymerase chain reaction to examine the survival of a human limbal stem cell allograft. Am J Ophthalmol 1995;120(3):342-50.
9. Henderson TR, Coster DJ, Williams KA. The long term outcome of limbal allografts: the search for surviving cells. Br J Ophthalmol 2001;85(5):604-9.
10. Henderson TR, Findlay I, Matthews PL, et al. Identifying the origin of single corneal cells by DNA fingerprinting: part I – implications for corneal limbal allografting. Cornea 2001;20(4):400-3.
11. Henderson TR, Findlay I, Matthews PL, et al. Identifying the origin of single corneal cells by DNA fingerprinting: part II – application to limbal allografting. Cornea 2001;20(4):404-7.
12. Henderson TR, McCall SH, Taylor GR, et al. Do transplanted corneal limbal stem cells survive in vivo long-term? Possible techniques to detect donor cell survival by polymerase chain reaction with the amelogenin gene and Y-specific probes. Eye 1997;11(Pt 6):779-85.
13. Daya SM, Watson A, Sharpe JR, et al. Outcomes and DNA analysis of ex vivo expanded stem cell allograft for ocular surface reconstruction. Ophthalmology 2005;112(3):470-7.
14. Pellegrini G, Ranno R, Stracuzzi G, et al. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation 1999;68(6):868-79.
15. Munster AM. Cultured skin for massive burns. A prospective, controlled trial. Ann Surg 1996;224(3):372-5; discussion 375-7.
16. Odessey R. Addendum: multicenter experience with cultured epidermal autograft for treatment of burns. J Burn Care Rehabil 1992;13(1):174-80.
17. Cairns BA, deSerres S, Peterson HD, et al. Skin replacements. The biotechnological quest for optimal wound closure. Arch Surg 1993;128(11):1246-52.
18. Choucair MM, Phillips TJ. What is new in clinical research in wound healing. Dermatol Clin 1997;15(1):45-58.
19. Nguyen TT, Gilpin DA, Meyer NA, et al. Current treatment of severely burned patients. Ann Surg 1996;223(1):14-25.
20. Munster AM. Whither [corrected] skin replacement? Burns 1997;23(1):v.
21. Chen JJ, Tseng SC. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Invest Ophthalmol Vis Sci 1991;32(8):2219-33.
22. Chen JJ, Tseng SC. Corneal epithelial wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci 1990;31(7):1301-14.
23. Huang AJ, Tseng SC. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci 1991;32(1):96-105.
24. Kruse FE, Chen JJ, Tsai RJ, et al. Conjunctival transdifferentiation is due to the incomplete removal of limbal basal epithelium. Invest Ophthalmol Vis Sci 1990;31(9):1903-13.
25. Tseng SC. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology 1985;92(6):728-33.
26. Tseng SC. Topical retinoid treatment for dry eye disorders. Trans Ophthalmol Soc UK 1985;104(Pt4):489-95.
27. Shimmura S, Tsubota K. Surgical treatment of limbal stem cell deficiency: are we really transplanting stem cells? Am J Ophthalmol 2008;146(2):154-5.
28. Donisi PM, Rama P, Fasolo A, et al. Analysis of limbal stem cell deficiency by corneal impression cytology. Cornea 2003;22(6):533-8.
TAKE HOME MESSAGE
-
The clinical features of LSCD have potential explanations other than deficiency of progenitor cells.
-
Chronic and damaging signals can drive the cells to undergo phenotypic alterations that can reverse when the injurious agent is removed and inflammation eliminated. This would allow cells to return to their normal state and we speculate a proportion of cases treated successfully as stem cell deficiency may be cases of ocular surface metaplasia.
-
The adjunctive treatment such as systemic immune-suppression and amniotic membrane transplant may have restored the corneal surface to normality. This concept may explain why, in the absence of engraftment, normality was restored.
Declaration of Competing Interests: None declared.
COMMENTS ARE WELCOME