The author shares his experience of setting up a nurse-led service to deliver anti-VEGF intravitreal injections and how injection assistant devices supported training.
Age-related macular degeneration (AMD) has become a leading cause of irreversible blindness [1]. It is estimated that over 20% of the populations in developed nations may now have this condition owing to an ageing population demographic [2]. Neovascular AMD (nAMD) is an aggressive form which has often resulted in significant visual impairment [3]. Fortunately, the discovery of intravitreal anti-vascular endothelial growth factor (anti-VEGF) for treatment of nAMD, has dramatically improved the visual prognosis for this group of individuals. The ANCHOR and MARINA studies have found that 33% of patients receiving monthly Ranibizumab 0.5mg intravitreal injections demonstrated moderate visual gain (≥15 letters) and 95% avoided moderate visual loss (≤15 letters) [4].
The number of individuals requiring anti-VEGF intravitreal injections has increased exponentially. Consequently, new challenges, such as how to accommodate these individuals into an already stretched ophthalmology service, have arisen nationally. This resulted in the Royal College of Ophthalmologists issuing advice in 2013 advocating the training of non-medical professionals, such as qualified nurses, on performing intravitreal injections independently [6,7]. The Professional Standards Committee of the College in its guidance for the use of such healthcare professionals (HCP) to deliver intravitreal injections, highlighted the importance of maintaining the highest degree of ethical standards and safety [7].
The use of non-medical professionals to deliver intravitreal injections has been shown to be safe and feasible in multiple ophthalmology departments in the UK [9,10,11]. A recent systematic review including 31,303 intravitreal injections delivered by 16 nurses found comparable complication rates: post-injection endophthalmitis incidence ranged from 0-0.40% for nurses and 0.0-0.50% for doctors [12]. The next question posed is how to streamline the training of non-medical professionals to increase capacity quickly and safely?

Figure 1: Intravitreal injection assistant device (InVitria) in use.
Pre-filled syringes for ranibizumab treatment have been developed to reduce preparation time and variation in preparation steps [13]. In addition, injection assistant devices such as the InVitria® (FCI Ophthalmics, USA) and SP.eye™ (Surgitrac Instruments, UK), have been developed to aid with identifying injection location (Figure 1), further reducing the preparation time and preventing the injecting needle from coming into contact with the eyelashes and eyelid [14]. InVitria® is made from a transparent polycarbonate mould that fits around the cornea with a central window allowing for patient fixation. There is a 28-degree-angled, guide tube which the needle passes through, delivering the injection at a fixed distance of 3.5mm from the limbus [14].
We report on the use of InVitria® to streamline the training programme for nursing staff learning to deliver anti-VEGF intravitreal injections in the context of a UK district general hospital. We also discuss the challenges encountered in the setting up of this service.
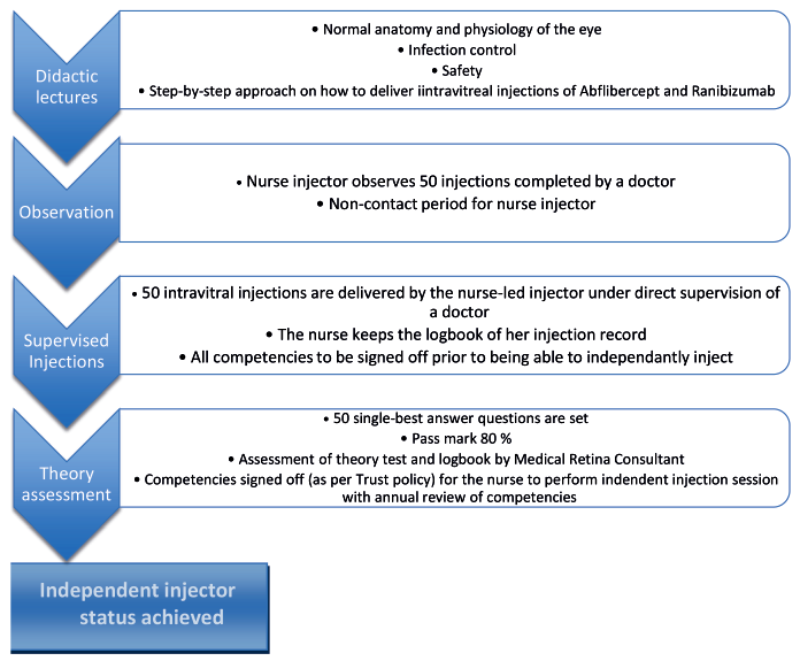
Figure 2: Outline of training regime received by training nurse-led
injectors prior to acquiring independent injector competencies.
Nurse-led injector training programme
Training of nurse practitioners to perform intravitreal injections at the Great Western Hospital consists of the following components (Figure 2):
a. Theory course: the trainee nurse is provided with a hand-out that contains basic information about the anatomy of the eye, pathology of age-related macular degeneration and the therapeutic action of anti-VEGF agents. The course also contains theoretical aspects of delivering the injections.
b. Observation: the nurse then observes a minimum of 50 intravitreal injections administered by a doctor over four to five injection clinics.
c. Injecting under supervision: a minimum of 50 injections are administered by the nurse being supervised by a doctor, all nurses are trained in using InVitria® in administering injections, when this part of training is successfully completed the nurse proceeds for the final assessment.
d. Theory test: this consists of 50 single best answer questions, only when achieving the pass mark of 80% is the nurse signed-off to carry out injection sessions independently.
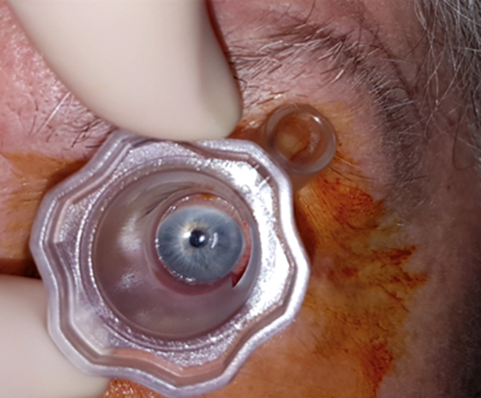
Figure 3: InVitria orientation mark lined-up.
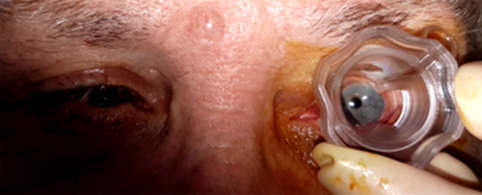
Figure 4: Gentle pressure keeps the eye fixed.
The injection process
After identifying the patient and confirming the side of injection and the drug to be injected, Proxymetacaine 0.5% local anaesthetic drops are instilled twice into the eye to be injected. The eye is cleaned with Povidone Iodine 5% and the InVitria® device is placed on the patient’s eye with the central orientation mark correctly lined up (Figure 3). A gentle downward pressure with a 45-degree turning force ensures the eye becomes fixed in place (Figure 4); alternatively, if the visual acuity is good the patient is asked to look at a certain spot. The patient is then unable to see the needle. The nurse injector inserts the needle into the eye using the guide tube of the InVitria® device, which has a pre-set angle, depth and distance from the limbus of 3.5mm, and the drug is injected into the eye. The needle is removed and the InVitria® device removed using a reversed 45-degree turning movement. This results in a stepped injection puncture due to the displacement of the conjunctiva. Topical Chloramphenicol 0.5% and Sodium hyaluronate 0.4% preservative free drops are instilled to the eye following injection.
Table 1: Cost analysis
Item: InVitria® device
InVitria® Unit price: 7.50
Standard pack: None
Item: Dressing pack
InVitria® Unit price: 0.44
Standard pack: I Vit pack
Item: Cotton bud
InVitria® Unit price 0.02
Standard pack: I Vit pack
Item: Hand towel
InVitria® Unit price 0.09
Standard pack: I Vit pack
InVitria® Unit price: Total: 8.05
Standard pack: Total: 16.08
Cost analysis
Table 1 shows cost break down for intravitreal injections standard pack compared to the InVitria® device, the saving achieved by using InVitria® is more than 50% of the cost. This becomes more significant when the overall number of injections per list is considered, we managed to increase the number of injections per list to 16 in average (from 13 injections per list) with a total reduction of the cost from £257.78 (16.08x16) down to £128.8 (8.05x16). This cost propagates to become even more obvious when the total number of injection lists is considered, we are now able to run more injection lists as more nurses use InVitria® to carry out injections and patients are significantly happier with the results (Hasan H, et al.) so the yearly saving by using InVitria is £45,000. Added to the savings is the revenue generated by increasing the capacity, the total number of injections per list multiplied by the weekly number of injections per week by 44 working weeks per year has increased from £377,530 to £619,200.
Discussion
We have successfully trained five nurses to perform intravitreal injections independently using the InVitria® device to facilitate injections. The project started with one staff nurse in 2014 and the training scheme was piloted to highlight any issues and resolve them on a small scale prior to initiating further training of ophthalmic staff nurses. The average time for this individual to be a fully competent injector was six months, at which point they had completed over 200 injections.
Following this training period, it took a further six months to establish fully functional nurse-led clinics and ensure all clerical processes were completed. Following this pilot scheme, four more nurse injectors were trained. This took on average 10 weeks for training to be completed with 50 injections completed under supervision by each nurse injector. This second wave of training took a significantly shorter time to complete, this is largely because the training modules were fully developed and the use of InVitria® as an assisting device was established, in addition to the administrative processes which were already in place. Since initiating this project, the first nurse injector has successfully completed over 1250 intravitreal injections without significant complications.
There are multiple learning points which have arisen from running this training programme. These maybe of use for other units developing their own nurse-led injector training programmes. We believe it is important to have a pilot phase with a single nurse being trained as an injector so any issues can be resolved on a small-scale. Specifically, we encountered a delay in organising nurse-led training clinics as this required a doctor to be present for at least eight clinics in total and reassigned from other clinical duties. Therefore, it is important to anticipate changes to clinical duties in advance and plan for assignment of a doctor to at least eight injection clinics per nurse injector. Initially it was difficult to recruit the first nurse injector. Improved transparency on what to expect and detailed consultation on the injection process may have helped with this in the first instance, as the second wave of training was met enthusiastically by more than the number of places available.

Figure 5: The medical retina team at the Great Western Hospital.
We also have two administrative staff members who are specifically responsible for coordinating all intravitreal injections and follow-up appointments. This has ensured a smooth transition from the initial training phase where only eight to ten patients are booked per list, to the independent nurse-led injector clinics with 16 patients booked per clinic.
For ease of training we chose to use the InVitria® device to facilitate the administration of all intravitreal injections. This device received good feedback from all five nurse injectors, who report that InVitria® is easy to handle and easy to get used to. In particular, it has been useful in stabilising the eye prior to injection, ensuring the correct location and position of the injection. Ultimately this removes the need to use a sterile drape, or measure and mark the site, resulting in a quicker, safe and more cost-effective injection process. We obtained feedback from the patient about InVitria®, there have been no issues reported. Some of the nurse injectors have reported that patients with deep-set or small eyes are often more difficult to apply the device. Specifically, the device can induce squeezing of the eye against it, which theoretically may increase the risk of abrasion when removing it.
References:
1. Pascolini D, Mario SP, Pokharel GP, et al. Global update of available data on visual impairment: a compilaton of population-based prevalence studies. Ophthalmic Epidemiol 2004;11:67‐115.
2. Lim L, Mitchell P, Seddon J, et al. Age-related macular degeneration. The Lancet 2012;379(9827):1728-38.
3. Wong TY, Wong T, Chakravarthy U, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology 2008;15:116-26.
4. Johnston RL, Lee AY, Buckle M, et al. UK Age-Related Macular Degeneration Electronic Medical Record System (AMD EMR) Users Group. Report IV: incidence of blindness and sight impairment in ranibizumab-treated patients. Ophthalmology 2016;123:2386-92.
5. Chin-Yee D, Eck T, Fowler S, et al. A systematic review of as needed versus treat and extend ranibizumab or bevacizumab treatment regimens for neovascular age-related macular degeneration. Br J Ophthalmol 2016;100:914-7.
6. Amoaku W, Blackeney S, Freeman M, et al. Action on AMD. Optimising patient management: act now to ensure current and continual delivery of best possible patient care. Eye 2012;26(Suppl 1):S2-S21.
7. Kirkby G. College Statement on intra-ocular injections by non-medical health care professionals (HCPs).
https://www.rcophth.ac.uk/wpcontent/uploads/
2014/12/2013_PROF_221_College_
Statement_on_intravitreal_injections.pdf
Last accessed December 2017.
8. DaCosta J, Hamilton R, Nago J, et al. Implementation of a nurse delivered intravitreal injection service. Eye 2014;28:734-40.
9. Michelotti M, Abugreen S, Kelly SP, et al. Transformational change: Nurses substituting for ophthalmologists for intravitreal injections – a quality-improvement report. Clin Ophthalmol 2014;8:755-61.
10. Simcock P, Kingett B, Mann N, et al. A safety audit of the first 10 000 intravitreal ranibizumab injections performed by nurse practitioners. Eye (Lond) 2014;28:1161-4.
11. Varma D, Lunt D, Johnson P et al. A novel approach to expanding the role of nurses to deliver intravitreal injections for patients with age-related macular degeneration. Int J Ophthalmic Pract 2013;4:68-74.
12. Rasul A, Subhi Y, Sorensen L, Munch I. Non-physician delivered intravitreal injection service is feasible and safe – a systematic review. Dan Med J 2016;63(5):A5229.
13. Souied E, Nghiem-Buffet S, Leteneux C et al. Ranibizumab prefilled syringes: benefits of reduced syringe preparation times and less complex preparation procedures. Eur J Ophthalmol 2015;25:529-34.
14. Ratnarajan G, Nath R, Appaswamy S et al. Intravitreal injections using a novel conjunctival mould: a comparison with a conventional technique. Br J Ophthalmol 2013;97:395-7.
Acknowledgment:
The author would like to thank Selina Khan, Shahina Ahmed, Waseem Qureshi, Anzela Makovej and Nimish Shah for their assistance in completing this article.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME