Diabetic macular oedema (DMO) is a major cause of visual loss in diabetes, with a complex multifactorial pathogenesis. In the UK alone it is estimated that there are nearly 2.5 million diabetic patients aged over 12 years.
Approximately 65,000 of these have clinically significant DMO that affects their visual acuity in at least one eye [1]. In DMO, the final common pathway is disruption of the blood-retinal barrier (BRB), resulting in leakage of fluid into the retinal layers, but causation is complicated, with many factors contributing to the process. Nevertheless, evidence is mounting that inflammation is implicated in BRB breakdown, together with hypoxia, alterations in blood flow and retinal ischaemia.
What role does inflammation play?
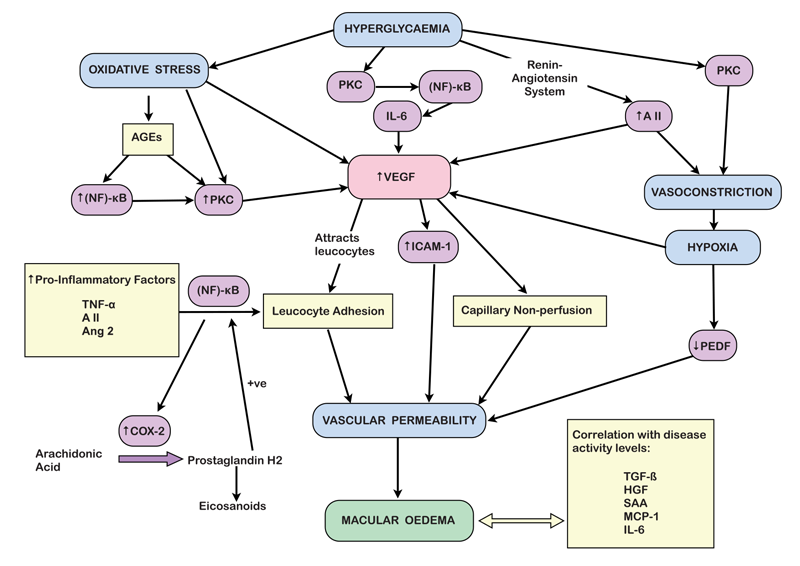
The inflammatory process underlying BRB dysfunction involves the activation of vascular endothelial growth factor (VEGF), alterations in endothelial intercellular junctions, retinal vessel leucocyte adhesion, decreased pigment epithelium-derived factor (PEDF) levels and activation of protein kinase C (PKC) [2]: together, these result in increased vascular permeability.
Hyperglycaemia feeds into the inflammatory process as it not only causes oxidative stress [3], but also leads to the formation of advanced glycation end products (AGEs). Animal studies have shown these AGEs to be capable of activating the inflammatory processes underlying DMO [4]. Chronic hyperglycaemia also indirectly leads to activation of PKC, and angiotensin II via the renin-angiotensin system, generating vasoconstriction and consequent hypoxia, and stimulating the production of the pro-inflammatory cytokine IL-6. Oxidative damage, angiotensin II and IL-6 all upregulate VEGF production [2,5].
Which inflammatory mechanisms are important?
VEGF is a particularly important factor in the pathogenesis of DMO and, as a result, it has become a major therapeutic target. It is one of a family of growth factors and is produced in the diabetic retina mainly by Müller cells, but also by ganglion cells, glial cells, retinal pigment epithelium (RPE) cells, pericytes, endothelial cells, and neurons [6]; hypoxia causes up-regulation of VEGF in all of these retinal cells. It is thought that retinal VEGF attracts leucocytes into the retinal vasculature, particularly monocytes / macrophages, which are active in diabetic retinopathy and which are known to migrate in response to VEGF [7].
Intercellular adhesion molecule-1 (ICAM-1) is another potential key player in the inflammatory process. It is a transmembrane extracellular glycoprotein that regulates the adhesion of circulating leucocytes to other resident cells. In rat models, it has been shown that VEGF promotes ICAM-1 up-regulation, leucocyte adhesion, vascular permeability and capillary non-perfusion [8]. VEGF and ICAM-1 both have a significant influence on vascular permeability and both have been positively correlated with the severity of DMO [9]. The kinin-kallikrein system may also be involved in the inflammatory process, being a central component of the innate inflammation pathway, however, its role in the diabetic eye is as yet incompletely understood.
Other inflammatory factors have also been implicated in the development of DMO. Transforming growth factor (TGF-β) [10], hepatocyte growth factor (HGF) [11], serum amyloid A (SAA) [12], monocyte chemoattractant protein-1 (MCP-1) and interleukin-6 (IL-6) [8] have all been identified in the ocular fluid of patients with DMO and can be correlated with levels of disease activity [13]. Other reports have also correlated an increase in VEGF levels to up-regulation of other pro-inflammatory factors such as tumour necrosis factor-α [14], angiotensin II [15], and angiopoietin 2 [16]. The resultant increase of adhesion molecules on the retinal endothelial cells is mediated by activation of transcription factor (NF)-κB [17], which then up-regulates cyclooxygenase (COX)-2 expression [18]. COX-2 is a key enzyme in the conversion of arachidonic acid to prostaglandin H2, the common precursor for all other eicosanoids and is expressed at sites of acute inflammation, creating a positive feedback loop by further activating (NF)-κB and other inflammatory cytokines [19,20].
Figure 1: Pathway for formation of diabetic macular oedema.
How can inflammation be targeted?
Corticosteroids act on a variety of mediators at different levels of the inflammatory cascade. Additionally, steroids have anti-angiogenic and BRB-stabilising abilities. They act by binding to a glucocorticoid receptor in the cytoplasm of target cells, thus regulating the transcription of certain genes including those encoding cytokines involved in the inflammatory process such as IL-1β, IL-2, IL-6, TNF‑α, GM-CSF and chemokines such as IL-8, MCP-1, MIP-1α and eotaxin [21]. Corticosteroids also exert an inhibitory effect on enzyme induction, thereby indirectly inhibiting the synthesis of several inflammatory mediators.
This results in a multi-faceted approach to the treatment of DMO, in contrast to anti-VEGF agents which act on only one specific factor in the inflammatory process. This is illustrated in studies, such as that conducted by Wen and co-workers who compared the effects of intravitreal triamcinolone and bevacizumab on cytokine levels in the aqueous humour, finding that bevacizumab affected only VEGF levels. However, intravitreal steroid not only suppressed the expression of VEGF but also multiple other inflammatory agents indicated in the development of DMO [22]. The clinical impact of this in the treatment of DMO still remains unclear.
How does this influence treatment?
The importance of VEGF in the inflammatory process has made it an ideal target for pharmacological intervention. The introduction of anti-VEGF agents administered by intravitreal injection, such as ranibizumab (Lucentis, Genentech Inc., San Fransisco, CA), bevacizumab (Avastin, Genentech Inc., San Fransisco, CA) and aflibercept (Eylea, Regeneron Pharmaceuticals Inc., NY), has increased the options available to both clinician and patient and has revolutionised the treatment of DMO. The role of corticosteroids is less clear, mainly as their use has hitherto been limited by their ocular side-effect profile.
Triamcinolone (Kenalog, Bristol Myers Squibb), dexamethasone (Ozurdex, Allergan Inc., NJ) and fluocinolone acetonide, (Iluvien, Alimera Sciences Inc., GA) are steroids currently available for intravitreal use, although triamcinolone is unlicensed for use within the eye in Europe (an approved preparation called Triessence exists in the US, however). The latter two are sustained release preparations that dispense the drug at a controlled rate over a period of six months (Ozurdex) or two to three years (Iluvien). However, these agents come at the price of side-effects commonly associated with prolonged ocular steroid use: elevated intraocular pressure and cataract formation. It is for this latter reason that National Institute for Health & Care Excellence (NICE), in its guidance, has restricted the use of both Iluvien and Ozurdex to pseudophakic eyes only.
Evidence for the use of triamcinolone in DMO
The use of triamcinolone in DMO was appraised in the Diabetic Retinopathy Clinical Research Network (DRCR) protocol I randomised multicentre study, involving 854 eyes of 691 patients. Intravitreal 4mg triamcinolone (IVTA) or 0.5mg ranibizumab, plus focal / grid laser was compared with focal / grid laser alone for treating centre-involving DMO. One-year primary outcomes revealed that, in pseudophakes, IVTA and prompt laser appeared comparable to ranibizumab combined with prompt or deferred laser at improving visual acuity and reducing retinal thickening [23]. These results were maintained through year two.
In the treatment of DMO refractory to focal laser therapy, reviewed by Yilmaz et al, IVTA was found to give a greater improvement in VA at three months than either sub-Tenon triamcinolone or no treatment; however, this benefit did not persist to six months [24]. A similar pattern emerged for central macular thickness, with the benefit of IVTA at three months no longer being statistically significant at six months. In another study, looking into the treatment of diffuse DMO in 126 eyes of 126 patients, IVTA was compared with modified grid laser therapy and bevacizumab. The result showed triamcinolone to be as effective as bevacizumab, and better than laser, at improving visual acuity (VA) and central macular thickness at six months. By 12 months, VA stabilisation between the three groups was comparable to within ±0.2logMAR of baseline best corrected visual acuity (BCVA) [25]. In all of these studies, it was noted that there was an increased risk of intraocular pressure rise in the triamcinolone group.
Evidence for the use of Ozurdex in DMO
The efficacy of Ozurdex was investigated in the MEAD study [26], a three-year, randomised trial of dexamethasone sustained-release intravitreal implant against sham. The study looked at two different doses of dexamethasone implants (0.7mg and 0.35mg). Patients were included if they had had previous laser or medical treatment for DMO, provided that they had not received an intravitreal anti-VEGF agent within three months, nor triamcinolone within six months, of the start of the study. Treatment naïve patients were also included, as were phakic patients. Retreatment with dexamethasone implant was available at not less than six months from the previous implant. Overall, visual acuity gains were greater in patients receiving Ozurdex compared with sham, with a rapid onset in benefit that was sustained throughout the study period. Cataract development became an issue after the end of the first year of treatment, necessitating surgery in those affected.
The study was somewhat flawed in that a lack of rescue treatment within the trial meant that a significant proportion of the sham group were withdrawn during the trial (198/350). Retreatment was also not allowed at less than six month intervals.
In the treatment group, visual acuity declined at around three to four months, requiring re-treatment. Cataract developed in the second year of the study. Interestingly, in view of the multi-site action of steroid in opposing inflammatory processes, only 23 of the 351 patients enrolled on the dexamethasone 0.7mg arm of the trial, and 25 of the 347 patients on the 0.35mg arm, withdrew due to lack of efficacy.
The rate of significant IOP increase (≥10mmHg from baseline or a measurement ≥35mmHg) in Ozurdex-treated subjects was 62%, but 60% of these did not require treatment and there was no evidence of a cumulative increase in IOP over time. Fewer injections were required than with anti-VEGF treatments, with an average of five injections over three years. The best outcome was obtained in pseudophakic patients and here results were similar to real-world outcomes with anti-VEGF therapy, with a gain of approximately six letters over the 36 months of the trial (cf. the RESTORE study, in which ranibizumab alone, or combined with laser, was compared with laser alone. At 36 months from baseline, visual acuity gains were greatest in the ranibizumab-treated groups. In these, 6.7 letters were gained in the group initially treated with ranibizumab plus laser during the first ‘core’ 12 months of the study (mean 6.0 injections); and 8.0 letters in the group initially receiving ranibizumab alone (mean 6.8 injections) [27]). In the MEAD study, the VA gain was achieved with a mean of only 4.1 injections in the Ozurdex group.

Figure 2: Mean change in best-corrected visual acuity from baseline in
the subgroup of patients with pseudophakic study eyes at baseline [25].
MOZART was a retrospective multi-centre study set up to analyse the efficacy of Ozurdex in treatment-naïve patients [28]. There was an increase in visual acuity at one month post treatment, but the effect plateaued at two to four months, before declining by month six. These results support the findings of MEAD, suggesting that the optimal retreatment interval might be three to four months.
Evidence for the use of Iluvien in DMO
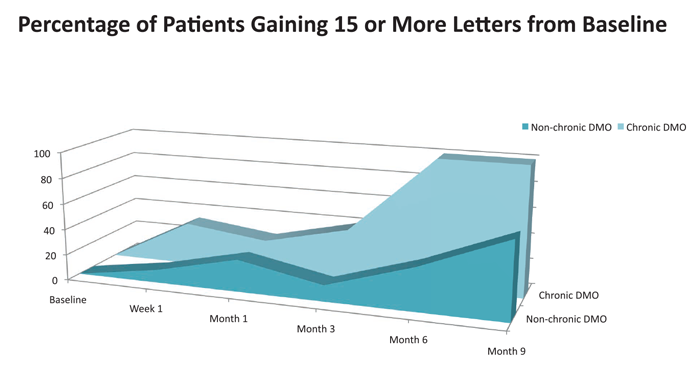
The FAME phase 3 clinical trials began in 2007 and compared two doses of Iluvien (fluocinolone acetonide, 0.2µg and 0.5µg) with sham over a three-year period to treat non-chronic DMO (<3 years’ duration) and chronic DMO (≥3 years’ duration) [29]. Of the 956 patients who were randomised 2:2:1 to 0.2µg/day and 0.5µg/day fluocinolone acetonide and sham, respectively, most (920 patients) had not been treated with anti-VEGF agents prior to entering the study. Rescue laser was permitted for all participants after six weeks’ treatment. The results reported were for the 0.2μg/day Iluvien vs. sham, as this dose represented the best risk-to-benefit ratio of the drug. The most notable finding was that greater improvements in visual acuity were seen in patients with chronic macular oedema (calculated by the study protocol as >3 years but in reality >1.73 years owing to an eccentric dating system). An increase in visual acuity of 15 letters or more from baseline was achieved in 32.8% of chronic DMO patients on Iluvien 0.2μg/day vs. 11.7% in the sham group, a treatment difference of 21.1%. Patients with non-chronic DMO fared less well.
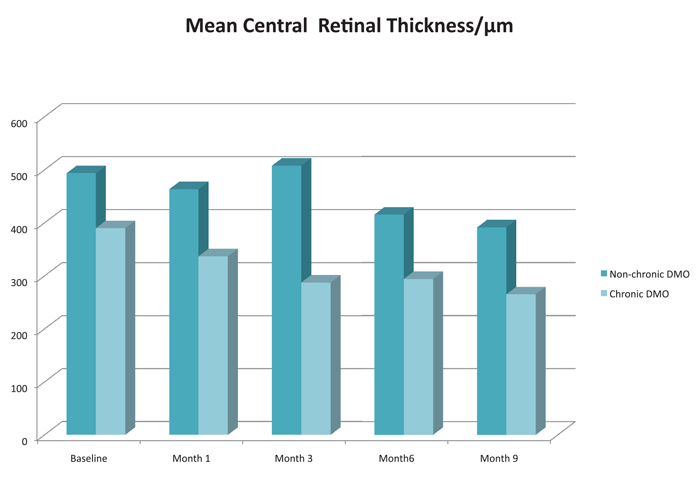
The pattern of results in the FAME study was echoed in a series of 21 eyes of 21 consecutive patients treated with Iluvien at the authors’ ophthalmology department. All of the patients were treated with the commercially-available 0.19µg/day dose of Iluvien, and none with sham. Nine-month results showed a greater mean improvement in visual acuity for patients with chronic DMO. Central retinal thickness scores were also better in the chronic DMO group than the non-chronic group. A major difference between our population and the FAME study cohort is that most of our patients (17 out of 21) had failed treatment with anti-VEGF prior to treatment with Iluvien implant, whereas anti-VEGF therapy was not yet standard-of-care at the commencement of the FAME study.
Figure 3: Percentage of patients gaining 15 or more letters from baseline.
Figure 4: Mean central retinal thickness / µm.
Where do we go from here?
Despite recent advances in our knowledge of the pathogenesis of DMO, it involves complex mechanisms that remain incompletely understood. Treatment with anti-VEGF agents is effective, but there is a significant proportion of non-responders (circa 30%) and the optimal treatment regime for these patients is unclear. Directing intervention at an underlying inflammatory pathogenesis is an intellectually attractive concept, but experience with intravitreal corticosteroids has been slightly disappointing thus far. In part, this is because outcome measures focus on visual acuity, and the results are confounded by the development of cataract. Nevertheless, it is clear that we need either a new corticosteroid preparation that is significantly less cataractogenic or to be able to target different elements of the inflammatory cascade more accurately and without resorting to corticosteroids.
In order to achieve this, novel therapeutic targets will be required. For instance, the plasma kallikrein kinin system (KKS) plays a central role in the innate inflammation pathway, and there is some evidence that it may be involved in the development of DMO. Although DMO can occur at any stage of pre-proliferative or proliferative retinopathy, it is more common in advanced diabetic retinopathy. With this in mind, it is interesting to note that components of the plasma KKS linked to inflammatory pathways, including prekallikrein (PK), have been identified in the vitreous in advanced diabetic retinopathy [29], although the mechanism for this is not well understood. PK, a serine protease, has been shown to induce vasogenic oedema in other organs [30], and inhibition of the KKS with pharmacological agents has been used to treat acute attacks of hereditary angioedema [31]. Further research into the role of the KKS in the development of DMO would be desirable. It remains to be seen whether it will become a future target for the development of pharmacological treatment of DMO.
References
1. Minassian DC, Owens DR, Reidy A. Prevalence of diabetic macular oedema and related health and social care resource use in England. Br J Ophthalmol 2012;96(3):345-9.
2. Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophth 2009;54:1-32.
3. Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res 2003;22(1):1-329.
4. Ramasamy R, Vannucci SJ, Yan SS, et al. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration and inflammation. Glycobiology 2005;15(7):16R-28R.
5. Cohen T, Nahari D, Cerem LW, et al. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem 1996;271(2):736-41.
6. Famiglietti EV, Stopa EG, McGookin ED, et al. Immunocytochemical localization of vascular endothelial growth factor in neurons and glial cells of human retina. Brain Res 2003;969(1-2):195-204.
7. Barleon B, Sozzani S, Zhou D, et al. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 1996;87(8):3336-43.
8. Miyamoto K, Khosrof S, Bursell SE, et al. Vascular endothelial growth factor (VEGF) –induced retinal vascular permeability is mediated by intercellular adhesion molecule-1 (ICAM-1). Am J Pathol 2000;156(5):1733-9.
9. Funatsu H, Noma H, Mimura T, et al. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology 2009;116(1):73-9.
10. Forooghian F, Kertes PJ, Eng KT, et al. Alterations in the intraocular cytokine milieu after intravitreal bevacizumab. Invest Ophthalmol Vis Sci 2010;51:2388-92.
11. McAuley AK, Sanfilippo PG, Hewitt AW, et al. Vitreous biomarkers in diabetic retinopathy: a systematic review and meta-analysis. J Diabetes Complications 2014;28:419-25.
12. Ma Y, Tao Y, Lu Q, et al. Intraocular expression of serum amyloid a and interleukin-6 in prloferative diabetic retinopathy. Am J Ophthalmol 2011;152:678-85.e2.
13. Funatsu H, Yamashita H, Noma H, et al. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol 2002;133(1):70-7.
14. Funatsu H, Yamashita H, Shimizu E, et al. Relationship between vascular endothelial growth factor and interleukin-6 in diabetic retinopathy. Retina 2001;21(5):469-77.
15. Funatsu H, Yamashita H, Ikeda T, et al. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with diabetic macular edema and other retinal disorders. Am J Ophthalmol 2002;133(4):537-43.
16. Watanabe D, Suzuma K, Suzuma I, et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol 2005;139(3):476-81.
17. Wallach D. Cell death induction by TNF: a matter of self control. Trends Biochem Sci 1997;22(4):107-9.
18. Lim JW, Kim H, Kim KH. Nuclear factor-kappaB regulates cyclo-oxygenase-2 expression and cell proliferation in human gastric cancer cells. Lab Invest 2001;81(3):349-60.
19. Pairet M, Engelhardt G. Distinct isoforms (COX-1 and COX-2) of cyclo-oxygenase: possible physiological and therapeutic implications. Fundam Clin Pharmacol 1996;10(1):1-17.
20. McCormack K. Roles of COX-1 and COX-2. J Rheumatol 1998;25(11):2279-81.
21. Barnes PJ. Anti-inflammatory actions of glucocorticoids:molecular mechanisms. Clin Sci 1998;94:557-72.
22. Elman MJ, Aiello LP, Beck RW, et al. Diabetic Retinopathy Clinical Research Network: Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular oedema. Ophthalmology 2010;117(6):1064-77.
23. Yilmaz T, Weaver CD, Gallagher MJ, et al. Intravitreal triamcinolone acetonide injection for treatment of refractory diabetic macular edema: a systematic review. Ophthalmology 2009;116(5):902-11.
24. Sobaci G, Ozge G, Erdurman C, et al. Comparison of grid laser, intravitreal triamcinolone, and intravitreal bevacizumab in the treatment of diffuse diabetic macular oedema. Ophthalmologica 2012;227(2):95-9.
25. Boyer DS, Yoon YH, Belfort R, et al. Three-year randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014;121(10):1904-14.
26. Mitchell P, Bandello F, Schmidt-Erfurth et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011;118(4):615-25.
27. Matonti E, Pommier S, Hajjar C, et al. MOZART Study: Sustained-release dexamethasone intravitreal implant in diabetic macular oedema. realites Ophthalmologiques 2014;213.
28. Cunha-Vaz J, Ashton P, Iezzi R, et al; for the FAME Study Group. Sustained delivery fluocinolone acetonide vitreous implants: long term benefit in patients with chronic diabetic macular oedema. Ophthalmology 2014;121(10):1892-903.
29. Gao BB, Clermont A, Rook S, et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med 2007;13:181-8.
30. Kaplan AP, Joseph K. The bradykinin-forming cascade and its role in hereditary angioedema. Ann Allergy Asthma Immunol 2010;104:193-204.
31. Zuraw B, Yasothan U, Kirkpatrick P. Ecallantide. Nat Rev Drug Discov 2010;9:189-90.
TAKE HOME MESSAGE
-
Anti-VEGF agents are a good first line pharmacological intervention for DMO.
-
Consider using an alternative anti-VEGF agent if there is an inadequate response to the first anti-VEGF drug.
-
Corticosteroids are useful in DMO refractory to anti-VEGFs, although there are the risks of raised IOP and cataract formation.
-
Trimacinolone is useful to test whether the patient’s DMO responds to steroid, and the likelihood of IOP rise, as it has a shorter duration of action than the sustained-release steroid preparations.
-
Iluvien is for use in pseudophakic eyes only, thus avoiding the risk of cataract formation.
Declaration of Competing Interests
Fiona Harris has been sponsored by Alimera Sciences Ltd for attendance at a conference.
COMMENTS ARE WELCOME