Treatment practice in the management of neovascular age-related macular degeneration (AMD) and diabetic macular oedema (DMO) illustrate increasing adoption of patient-tailored treatment approaches based on initial diagnosis and regular monitoring of imaging and visual outcomes in routine clinical care. The author considers related guidance and recent presentations by retina specialists reviewing clinical experience.
Flexible dosing regimens for neovascular AMD may strike a pragmatic balance
Too few injections and only infrequent monitoring in busy clinical practice are often cited as possible explanations for long-term vision outcomes that fall short of results observed in rigorous pivotal clinical trials in neovascular AMD. Administering antiangiogenic injections continuously on a monthly or bimonthly pro re nata (PRN) regimen with strict regular monitoring has been found to produce the optimum results in vision outcome for neovascular AMD. For everyday practice, however, using an individualised PRN or a flexible treat and extend injection regimen for neovascular AMD may help achieve a better balance of retreatment need to ensure maximum visual gain long term while at the same time minimise clinic visits and avoid overtreatment.
“The challenge when adopting a PRN treatment regimen is that markers for disease activity are poorly defined.”
The standard of care for treating neovascular AMD is antivascular endothelial growth factor (anti-VEGF) therapy using intravitreal ranibizumab (Lucentis, Novartis) or aflibercept (Eylea, Bayer). The European label for ranibizumab in treating neovascular AMD recommends that treatment is initiated with one injection per month until maximum visual acuity (VA) is achieved and / or there are no signs of disease activity, i.e. no change in visual acuity and in other signs and symptoms of the disease under continued treatment. Thereafter, monitoring and treatment intervals should be determined by the treating physician and should be based on disease activity, as assessed by visual acuity and / or anatomical parameters. The treatment interval should be extended by no more than two weeks at a time for wet AMD. Aflibercept treatment for neovascular AMD is initiated with one injection per month for three consecutive doses, followed by one injection every two months for the remainder of year one. After the first 12 months, the treatment interval may be extended on the basis of visual and anatomic outcomes.
A treat and extend intravitreal injection regimen involves treating and then extending the interval until the next treatment by two-week intervals, provided the disease remains inactive. The rationale for individualised treat and extend is to reduce the treatment burden by avoiding unnecessary treatment in patients who have successfully achieved inactive disease. If disease activity is detected, treatment is given and the interval to the next treatment shortened.
Results from the 2015 Global Trends in Retina Survey show that in the United States, most practitioners (66.2%) generally treat neovascular AMD patients with active choroidal neovascularisation (CNV) using a treat and extend approach (treat until dry on optical coherence tomography (OCT), then extend treatment interval), whereas in Europe, 40.6% follow an as needed approach (treat until dry on OCT, then as needed) and 30.4% treat and extend [1].
Long-term outcomes suggest undertreatment is common
Practitioner experience and clinical expert opinion on treatment strategies for neovascular AMD were reviewed at the 2015 annual meeting of the American Society of Retina Specialists (ASRS) in Vienna, Austria. The challenge when adopting a PRN treatment regimen is that markers for disease activity are poorly defined, commented specialists. Moreover, poor initial diagnosis and low treatment frequency in neovascular AMD patients with active disease may explain observations of real-world outcomes that are at times less favourable than clinical trial results. Many clinicians believe that undertreatment is the principal reason, pointing to evidence showing the presence of persistent fluid, as well as poor treatment adherence or compliance with monitoring visits.
The Pan-American Collaborative Retina Study (PACORES) Group has studied the five-year anatomical and functional outcomes of 247 (292 eyes) patients with CNV secondary to AMD treated with unlicensed intravitreal bevacizumab. Mean follow-up was 68.1±8.9 months (60 to 120 months), and the average numb/Users/creative5/Desktop/YourGiftCard.pdfer of injections administered was 10.9±6.4 (one to 46 injections) over five years. Results show that most of the vision gains in vision and central macular thickness reduction are lost at 60 months’ follow-up. Diminished therapeutic response over time after repeated administration of the drug, natural disease progression, or undertreatment are potential contributing factors for the observed failure to maintain initial early vision gains beyond two years.
George Parlitsis (Rush University Medical Center/Illinois Retina Associates) outlined results evaluating long-term outcomes in anti-VEGF treatment-naïve wet AMD patients treated with anti-VEGF therapy in a multi-physician practice setting (45 eyes of 33 patients with an average age of 74). Over a mean period of 6.2 years, study patients received an average of 23.4 injections, with an average 5.3 injections at six months and 9.6 injections at two years. The study demonstrates that anti-VEGF therapy for neovascular AMD improves visual acuity with sustained improvement during the first two years. Over time however, multiple factors lead to a decrease in VA with an average final VA lower than that of the initial visit. After approximately 6 years of anti-VEGF treatments, 55% of eyes maintained stable or improved vision, while 45% suffered a decline.
Too few injections, atrophy, scar formation or other causes related to the natural disease process were possible contributing factors.
Disappointing real-life data from an evaluation of treatment outcomes with intravitreal anti-VEGF therapy were outlined by Gerhard Kieselbach, from Innsbruck, Austria. In 2013, 54,321 intravitreal injections of anti-VEGF agents were performed, most administered in Austrian hospitals, and 16,562 eyes were identified (66% AMD, 22% DMO and 12% retinal vein occlusion). Visual acuity records from five eye centres for 6,547 intravitreal injections (1,238 eyes) showed VA improvement in 12%, vision stabilisation in 51% and a loss of VA letters in 37%. The medium number of injections administered was 3.3 per eye for one year (range 2.9 to 3.7).
Flexible dosing regimen for maximum benefit and minimum burden
Different treatment regimens using ranibizumab for neovascular AMD have been evaluated. Results from the HARBOR Study Group show that, at two years’ follow-up, mean change from baseline in best corrected visual acuity (BCVA) (letters) was +9.1 for ranibizumab 0.5mg monthly, +7.9 for 0.5mg PRN, +8.0 for 2.0mg monthly, and +7.6 for 2.0mg PRN [2]. Quadrupling the ranibizumab dose to 2.0mg did not lead to better vision, while ranibizumab monthly and PRN ranibizumab provided rapid and sustained reduction of central foveal thickness. Ninety-three percent of the ranibizumab 0.5mg PRN arm did not need monthly injections to maintain improved vision.
The ATLAS study is an ongoing investigator-sponsored trial evaluating aflibercept treat and extend therapy for neovascular AMD. Criteria for extension of the retreatment period are absence of a ≥5 letter loss, absence of macular fluid on spectral domain OCT, absence of new macular heamorrhage and absence of leakage on fluorescein angiography. Visual and anatomic improvements were well maintained over one year of follow-up, with around half or more of all patients on a treatment interval of 10 weeks or greater at one year, with a mean injection number of 8.0 (1.6).
To maintain the vision and anatomic gains while minimising the treatment burden, three different treatment approaches beyond the first year using aflibercept for neovascular AMD have recently been proposed by an expert roundtable panel: continue with fixed eight-weekly dosing for eyes with active disease but stable visual acuity; manage using a treat and extend regimen for eyes with inactive disease and stable VA (extending by 2-week intervals, to a maximum of 12 weeks); or a trial of monitoring without treatment, for example using ‘virtual clinics’ to improve capacity, may be appropriate for cases where there has been no disease activity for ≥3 consecutive visits, initiated at the end of year one or any time during year two [3].
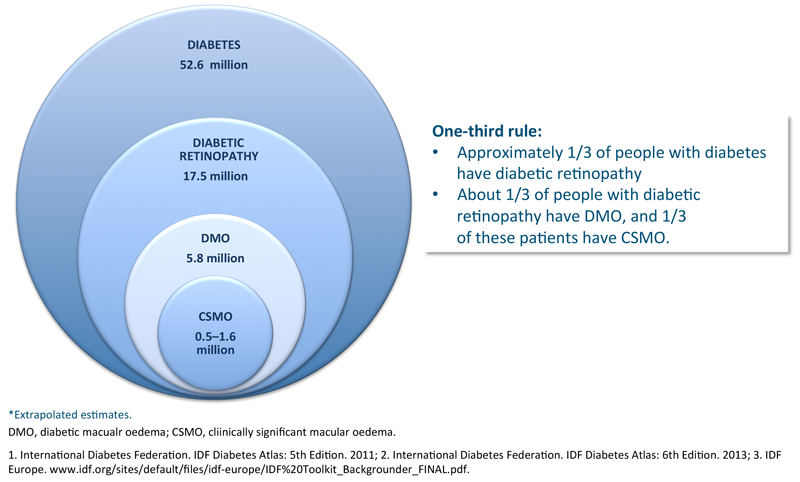
Figure 1: Diabetic eye disease, European epidemiology (2011)* 1.

Figure 2: Main causes of severe visual impairment certifications in England and Wales 2007-2008 (%) 1.
Diabetic macular oedema, individualised approach following recommended initiation regimen
Diabetic retinopathy is a common complication of diabetes which can lead to severe vision impairment (Figures 1 and 2). Treatment approaches that inhibit VEGF and block inflammation have been shown to improve visual outcomes for patients with macular oedema due to diabetes [4]. Moreover, prompt intervention has been shown to significantly lower the risk of severe vision loss in previously untreated DMO and chronic or recurrent DMO.
Antiangiogenic therapy and steroids approved by NICE and SMC for DMO
Guidance recommendations on the use of ranibizumab, fluocinolone acetonide intravitreal implant (Iluvien, Alimera Sciences), dexamethasone intravitreal implant (Ozurdex, Allergan) and aflibercept as possible treatment options for DMO have been issued by the National Institute for Health and Care Excellence (NICE) (Table 1).

Antiangiogenic agents ranibizumab and aflibercept are recommended treatment options for treating visual impairment due to DMO in eyes with a central retinal thickness (CRT) of 400µm or more at the start of treatment. Dexamethasone intravitreal implant was approved by NICE in 2013 as a therapeutic for DMO only in pseudophakic patients and the DMO does not respond to non-corticosteroid treatment, or such treatment is unsuitable. In 2013, fluocinolone acetonide intravitreal implant was approved by NICE as a treatment option for treating pseudophakic eyes having chronic DMO that is insufficiently responsive to available therapies.
For NHS Scotland, the Scottish Medicines Consortium (SMC) has recommended ranibizumab and aflibercept as options for treating visual impairment due to DMO in adults with BCVA of 75 letters or less at baseline, and fluocinolone intravitreal implant is recommended as a treatment option in chronic DMO considered insufficiently responsive to available therapies in pseudophakic eyes. The SMC has also recently accepted dexamethasone intravitreal implant for use by NHS Scotland for the treatment of adult patients with visual impairment due to DMO who are pseudophakic or who are considered insufficiently responsive to, or unsuitable for non-corticosteroid therapy.
Antivascular endothelial growth factor therapy is effective in treating visual impairment due to DMO, improving and maintaining visual acuity, with retreatment need declining significantly during the second and third years of treatment. The recommended dose for ranibizumab is 0.5mg, with treatment initiated with one injection per month until maximum visual acuity is achieved and / or there are no signs of disease activity. Thereafter, monitoring and treatment intervals are determined by the physician based on disease activity, assessed by visual acuity and / or anatomical parameters. If a treat and extend regimen is being followed, the treatment interval may be extended by up to one month at a time for DMO.
For the treatment of visual impairment due to DMO, aflibercept intravitreal injection is administered every month for five consecutive months, followed by one injection every two months with no requirement for monitoring between visits. After the first 12 months, the treatment interval may be extended based on visual and anatomic outcomes, with treatment discontinued if the patient is not benefiting from continued treatment.
In guidance recommendations issued for aflibercept in treating DMO, the NICE appraisal committee concluded that aflibercept was better than laser based on the results presented in pivotal clinical trials, and that aflibercept is likely to have similar clinical effectiveness to ranibizumab. The committee noted views from the Aberdeen Evidence Review Group that the evidence from the recently published DRCR.net Protocol T study was not relevant for their appraisal because the ranibizumab treatment arm was dosed at 0.3mg PRN (licensed dose in the United States), which is different to the dose specified in the European summary of product characteristics for ranibizumab, which is 0.5mg PRN. The committee concluded that, for people with a CRT of 400µm and more where ranibizumab is the comparator treatment, aflibercept is a cost-effective use of NHS resources for treating people with DMO. Clinical experts agreed that, based on clinical practice and clinical trial results, aflibercept is well tolerated and the committee accepted that there were no major safety concerns associated with aflibercept.
For patients treated with dexamethasone intravitreal implant, treatment is administered around every six months in the affected eye and up to seven implants may be given, providing an effective therapeutic option (over sham) for people with DMO who are unresponsive to prior therapy, or unsuitable for other regimens, while at the same time reducing the burden of multiple hospital visits. In clinical practice, DMO patients are typically treated with dexamethasone intravitreal implant more frequently than in the phase 3 clinical studies, with a median interval between implants of approximately four to five months.
Subthreshold laser therapy may be indicated in DMO patients who do not respond to pharmacologic therapy.

Figure 3: DRCR.net Protocol T trial - mean change in visual acuity letter score, full cohort.

Figure 4: DRCR.net Protocol T trial - mean change in visual acuity letter score, baseline visual acuity 20/50 or worse.

What did the DRCR.net Protocol T trial reveal at one year?
The DRCR.net Protocol T clinical trial found that aflibercept (dose 2.0mg), unlicensed bevacizumab (1.25mg) or ranibizumab (0.3mg) improved vision in eyes with centre-involved DMO at one-year follow-up, but the relative effect depended on baseline vision (Figures 3 and 4, Table 2) [5]. Few eyes in any group had substantial VA loss.
Overall, Protocol T showed that ranibizumab and aflibercept are superior to bevacizumab, while greater visual acuity improvement on average was seen with aflibercept than with the other agents. At poorer starting levels of vision, aflibercept had a clinically meaningful advantage: a gain in the VA letter score of at least 15 was observed in 63% more aflibercept-treated eyes than bevacizumab-treated eyes (67% vs. 41%), and in 34% more aflibercept-treated eyes than ranibizumab-treated eyes. Bevacizumab had a lesser effect on reducing macular oedema than the other agents, regardless of starting vision. Serious adverse event, death and hospitalisation rates appeared similar among treatment groups. No differences were noted in intraocular inflammation, and endophthalmitis was rare (0.02% of injections).
The European ranibizumab label recommends treatment at a dose of 0.5mg for treating visual impairment due to DMO (based on the RESTORE and RESOLVE studies). Some practitioners have argued that if a PRN posology is used, 0.5mg ranibizumab may be more efficacious than a 0.3mg ranibizumab dose for DMO. The US label states that compared to monthly ranibizumab 0.3mg for DMO, no additional benefit was observed with monthly treatment with ranibizumab 0.5mg (based on the RISE and RIDE clinical trials).
In long-standing DMO, an audit of one year outcomes from a sample of 53 patients (84 eyes) with chronic DMO treated with 0.5mg ranibizumab (three loading doses following by PRN retreatment) showed a mean VA change from baseline to month 12 of +7.2 letters (baseline mean VA 57 letters) and a mean of 6.89 injections in year one [6]. Mean CRT reduction from baseline to month 12 was 123µm, and with 24 eyes or 27.4% having a CRT of less than 250µm at one year compared with just one eye at baseline. This study found that DMO patients with baseline CRT of less that 400µm also benefited from intravitreal anti-VEGF treatment (33 eyes or 39.3% had baseline subfoveal CRT thickness <400µm).
Discussion
Different yet flexible dosing regimens using anti-VEGF therapy, for both neovascular AMD and DMO, may help avoid overtreatment or undertreatment, be more manageable in routine practice, and, subject to adequate monitoring, allow clinics to achieve good overall vision outcomes and reduce treatment burden where possible.
For DMO, retreatment need often diminishes with time, while neovascular AMD is a chronic condition characterised often by lifelong disease activity. The latter may require more aggressive monitoring and retreatment as needed if early and significant gains achieved on antiangiogenic therapy are to be sustained beyond year two. Recent evidence further confirms treatment benefits with VEGF inhibitor therapy in neovascular AMD patients with baseline vision better than 6/12 [7].
References
1. Renzaei KA, Stone TW, eds. 2015 Global Trends in Retina Survey. Chicago, IL. American Society of Retina Specialists; 2015.
2. Ho AC, Busbee BG, Regillo CD, et al; HARBOR Study Group. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014;121(11):2181-92.
3. McKibbin M, Devonport H, Gale R, et al. Aflibercept in wet AMD beyond the first year of treatment: recommendations by an expert roundtable panel. Eye (Lond) 2015;29 Suppl 1:S1-S11.
4. Calvo P, Abadia B, Ferreras A, et al. Diabetic macular edema: options for adjunct therapy. Drugs 2015 Aug [Epub ahead of print].
5. Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015;372(13):1193-203.
6. Jasani K, Walkden A, McKenna E, et al. Diabetic macular oedema treatment with ranibizumab. Novel method of audit of outcomes at one year. Poster presentation, annual congress of the Royal College of Ophthalmologists 2015, Liverpool UK; 19-21 May 2015.
7. Lee AY, Lee CS, Butt T, et al; UK AMD EMR Users Group. UK AMD EMR USERS GROUP REPORT V: benefits of initiating ranibizumab therapy for neovascular AMD in eyes with vision better than 6/12. Br J Ophthalmol 2015;99(8):1045-50.
TAKE HOME MESSAGE
-
Treatment approaches that inhibit vascular endothelial growth factor (VEGF) and block inflammation have been shown to improve visual outcomes for patients with macular oedema due to diabetes.
-
Overall, Protocol T showed that ranibizumab and aflibercept are superior to bevacizumab for DMO, while greater visual acuity improvement on average was seen with aflibercept than with the other agents.
-
Low treatment frequency in neovascular AMD patients with active disease may account for disappointing vision outcomes long term.
-
Using a flexible treat and extend regimen for neovascular AMD may help achieve a better balance of retreatment need to ensure maximum visual gain while at the same time minimising clinic visits and avoiding overtreatment
COMMENTS ARE WELCOME