Rod McNeil breaks down the impact of the Brexit deal on healthcare in the UK, including medicines regulation, research funding, sharing of information and the ability to work abroad.
A disorderly no-deal exit from the European Union (EU) was averted as both partners struck an eleventh-hour free trade deal on Christmas Eve last year that largely protects mutual interests, with a review planned in five years’ time (Figure 1) [1].

Figure 1: EU-UK Relations: from the UK referendum to a new Partnership Agreement.
Although wide-ranging, the trade deal is far from exhaustive and does not provide a full final set of rules for reciprocal trade and cooperation [2]. This leaves the door open for further coordination and discussions, leaving many hopeful that further bilateral partnership agreements will provide for improved outcomes going forward.
The bad news is that the deal will diminish not just Britain’s economic ties to the continent, but also security, educational and human connections, observed Charles Grant, Director of the Centre for European Reform [3]. He described the trade deal struck by Boris Johnson’s government with the EU as “thin and economically injurious”, predicting that the British will search at some point for ways of building a closer and more fruitful economic partnership.
Undoubtedly, the UK’s future and its place in Europe remain unsettled, at least for the foreseeable future.


The EU-UK Trade and Cooperation Agreement (TCA) governs the future trading and security relationship following the UK withdrawal from the EU (Table 1 and 2) [1]. It creates a new economic and social partnership, including transport, energy and mobility, and establishes a framework for law enforcement and judicial cooperation in criminal and civil law matters. Also included is an overarching horizontal agreement on governance, overseen by a Partnership Council, allowing for cross-sector retaliation (e.g., imposition of tariffs) across different economic areas in case of violations of the agreement (Figure 2).

Figure 2: Governance structure overseeing implementation of the EU-UK Trade and Cooperation Agreement.
While the trade agreement provides for zero tariffs and zero quotas on all goods that qualify, UK goods will need to meet rules of origin criteria to qualify for tariff-free access, i.e. exported products must have either been wholly obtained, or been subject to a significant amount of processing, in the EU or UK. The UK and EU will maintain separate regimes regulating human, plant and animal health. Both the UK and EU are obliged to carry out impact assessments of any changes to technical regulations. Both have agreed specific facilitations on medicinal products, motor vehicles, organics, wine and chemicals to streamline conformity assessment in these sectors.
Regulatory divergence for medicinal products and medical devices
Much to the dismay of the pharmaceutical and healthcare industry, in both Europe and the UK, the TCA does not contain a full Mutual Recognition Agreement (MRA), meaning that separate regulatory and oversight regimes will apply for marketing authorisations, CE marking, clinical trials and pharmacovigilance, for example.
“One of industry’s key asks was for both sides to reach a MRA on batch testing and inspections,” said a spokesperson for the Association of the British Pharmaceutical Industry (ABPI). “Whilst the deal saw an agreement on the recognition of inspections, it is still our position that the EU and UK should go further and agree a full MRA which covers both. The UK is currently unilaterally recognising EU batch testing, and we believe this should be reciprocated whilst a full MRA is worked out.”
Asked to comment on regulatory divergence and market access, a Medicines and Healthcare products Regulatory Agency (MHRA) spokesperson explained: “For medicines, Great Britain will operate to an initial legal framework that incorporates EU legislation into UK law. For devices, the existing provisions of EU law which are cross referred to in the Medical Devices Regulations 2002 are retained. For both medicines and medical devices, Northern Ireland will continue to follow to EU acquis under the terms of the Northern Ireland Protocol (NIP). However, the UK regulator will be able to take sovereign regulatory decisions to help evolve the UK legal regime to meet future UK needs, and to take regulatory decisions that will also apply directly in Northern Ireland, as long as those decisions do not conflict with EU decisions whilst the NIP applies.”
To help ensure continuity of supply of medicines and medical devices, the UK will unilaterally recognise certain EU regulatory processes for a time limited period.
One of the EU regulatory processes unilaterally recognised by the UK is automatic recognition of the decisions of the European Commission (EC) to authorise products under the Central Authorised Procedure (CAP) for two years. This will apply to most novel products and means companies who hold an EU Marketing Authorisation will be able to have their licence recognised by the MHRA and be provided with UK licences at a reduced fee with no additional assessment required. The working group on medicines will provide further guidance on this issue, which will be published in due course.
EU-UK future relationship: pharmaceutical industry perspective
Discussing the impact of Brexit and the EU-UK trade accord, Koen Berden, Executive Director, International Affairs, European Federation of Pharmaceutical Industries and Associations (EFPIA), commented in a telephone interview: “The general view is that Brexit is not helpful given the integrated supply chains that exist between the UK and what is now the EU27. The UK was an important partner in the ‘global system’ of the pharmaceutical industry, both for R&D as well as for manufacturing, but also for regulatory drug evaluation and approval. That is and remains a challenge, alongside continuing uncertainty around the degree of participation as ‘third country’ for the UK.
“In light of discussions that are taking place in Europe with respect to strategic resilience in the COVID-19 pandemic era, Brexit makes it more cumbersome to work between two partners who were previously much more integrated. On the positive side, we are pleased that the EU and UK concluded a last-minute free trade agreement, although the accord governing the future relationship lacks some important elements that we would like to see.
“The big negative is lack of a MRA on batch testing, despite long-standing equivalence recognition. The EU has struck MRAs on batch testing with several trading partners such as the US and Japan, waiving batch testing on import by manufacturers for medicines and vaccines. Erecting additional barriers between integrated European partners impacts our overall competitiveness versus the US and China, for example. Moreover, the requirement for additional batch testing may delay patient access to medicines.”
EFPIA welcomes the inclusion in the Brexit trade agreement of a pharmaceutical-specific annex, providing a platform for cooperation on wider policy issues regarding medicinal products, and the creation of a Working Group on Medicinal Products.
Mr Berden added: “We are happy with the living elements in the Brexit agreement and the commitment to explore further cooperation on regulatory standards and convergence. Our objective is to ensure that any potential disruptions to security of supply are minimised and, more generally, that unintended effects are resolved. Also, the phased-in approach for the Northern Ireland Protocol is important, as much-needed clarity is still lacking and it is important that the NIP is appropriately implemented.”
The NIP refers to the special conditions that allow Northern Ireland to continue benefiting from the EU’s single market for goods and customs union.
A key ask of EFPIA included continued alignment on data protection legislation between the EU and UK, access to relevant EU databases, including those supporting regulatory procedures, pharmacovigilance and security against falsified medicines, and a comprehensive sectoral adequacy assessment to support data transfers. While data adequacy is not included in the trade and cooperation agreement, a positive adequacy decision by the EU is expected.
Accelerating COVID-19 vaccine manufacturing capability and improving access globally to approved vaccines remain key challenges and, notes EFPIA, experience from 2020 underlines the importance of removing export restrictions, preventing stockpiling and opening borders to ensure the safe supply of medicines to patients across Europe. The establishment by the EC of an export authorisation system for COVID-19 vaccines risks delaying and could jeopardise the supply of vaccines across Europe and around the world, EFPIA said.
Health and life sciences sector: priorities ahead
Alexandre Regniault, Partner at international law firm Simmons & Simmons and head of the firm’s Healthcare and Life Sciences sector, considered in an interview: “The impact of Brexit on the European health products industry generally is considered negative. It is difficult at this time to envisage a positive aspect, given regulatory divergence and the lack of a mutual recognition agreement, as well as loss of British expertise within the European Medicines Agency (EMA). On a positive note, the industry has had time to prepare for the anticipated divergence, but, nonetheless, Brexit represents an additional burden and a source of uncertainty.
“On the positive front, the last-minute Brexit trade deal provides the foundation for better and more in-depth outcomes beyond the many non-binding provisions set out in the global agreement.”
Mr Regniault added: “In my opinion, priorities include addressing batch control, provision for a common foundation for intellectual property protection (including the Supplementary Protection Certificate system and Regulatory Data Protection) and market exclusivity for innovators. There is also no mutual recognition of notified bodes for evaluation and approval of medical devices, although EU CE marks will be valid through 30 June 2023.”
Continued participation in EU science funding programmes excellent for international collaborations
The EU-UK TCA provides for the UK’s association to Horizon Europe after the adoption of the Protocol on Programmes and activities in which the UK participates. Once the UK becomes an Associated Country, UK participants will have the same rights as EU participants in all calls open to their participation.
The EC expects association to become effective in time for the signature of the first grant agreements under the new programme. This means that UK entities will be eligible to participate in Horizon Europe from the start. Even if association is not yet formally effective, the first calls for proposals will ensure that UK applicants are treated as if they were based in an Associated Country throughout the process, from admissibility and eligibility, to evaluation, up until the signature of grant agreements, which can only be signed upon the association becoming effective.
Given the UK decision not to participate in any financial instruments in Horizon Europe, the scope of the UK association to the programme does not include the European Innovation Council (EIC) Accelerator Fund. This means UK entities can apply for grants under the Accelerator but they will not be eligible for loans or equity. They can also participate on equal footing with entities from EU Member States and other Associated Countries in EIC’s Pathfinder component.
Sir Richard Catlow, Vice-President and Foreign Secretary of the Royal Society, described the deal as ‘excellent news’, providing a pathway for ongoing and close scientific partnership between the UK and EU [4]. He added that the new UK Shared Prosperity Fund, which will replace EU structural funds, can play a valuable role in supporting the UK’s innovation landscape in the future. The end of UK association to Erasmus+ was described as “a sad casualty of the deal”.
Qualifications and mobility
There is no mutual recognition of professional qualification but the deal provides a route for mutual recognition to be agreed in the future and the UK and individual EU member states may strike bilateral agreements on the mutual recognition of professional qualifications.
Some 2268 European Economic Area (EEA) qualified doctors joined the General Medical Council’s register in the year to 30 June 2020, a rise of 7.5% over the previous year. Following Brexit, the registration process for some doctors with non-UK qualifications has changed. Doctors with a relevant qualification from the EEA will have those qualifications recognised for registration, but subject to that evidence being independently verified. The registration status of any doctor already holding provisional or full registration in the UK remains unchanged. For a UK qualified doctor who wants to work in the EEA, most EEA countries no longer automatically recognise qualifications from the UK now that the transition period has ended.
On a more positive front, the UK government’s ‘Global Health Insurance Card’, which will gradually replace the existing European Health Care Insurance Card, will continue to guarantee the rights of UK citizens to receive emergency and medically necessary healthcare when travelling in the EU.
The NHS Confederation welcomed news of the trade deal, as it “should offer a measure of much-needed certainty and security for the NHS, our partners and our patients” and lays the foundation for future agreements (Figure 3).
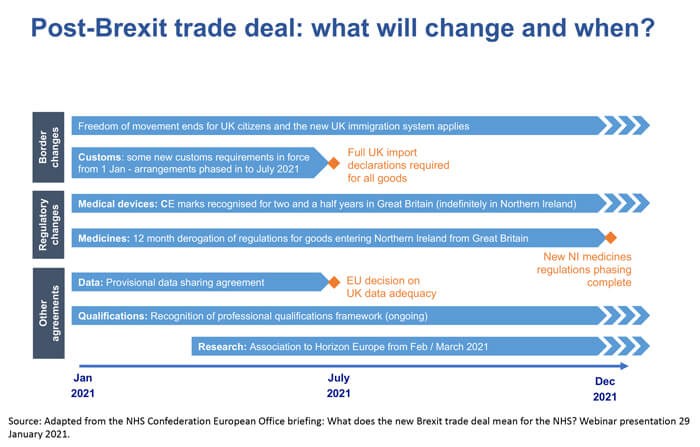
Figure 3: Post-Brexit trade deal: what will change and when?
Politicians encourage Brits to take the long view as realities of a hard Brexit kick in
The EU is by far the UK’s largest trading partner, accounting for almost half (48%) of total UK trade in 2019, with the UK trade deficit with the EU running at £80 billion a year. The terms of the trade deal and any future provisions will therefore play a large part in determining the economic impact of Brexit.
While the UK government has not published an impact assessment of the EU-UK trade deal, unlike for its recent accord with Japan, it believes that it’s a good deal that allows GB to maintain access to the EU market while securing GB sovereignty.
The Office for Budget Responsibility, based on analysis of external economic studies, estimated in November 2020 that a free-trade deal with the EU would leave Britain’s gross domestic product (GDP) 4% lower over the long run, compared with remaining in the bloc. Economic forecasts are grim, with GDP slumping by more than 10% last year and Britain facing a period of low growth compared with its peers [5].
“Britain will take several years to recover from Brexit and a worse-than-average COVID-19 experience,” opined Mr Grant from Centre for European Reform [3]. “It will be in permanent negotiation with the EU to improve the quality of their initially thin relationship. Yet if the country can overcome its Brexit culture wars, it has the potential to develop a successful global brand.”
References
1.European Commission DG Trade. The EU-UK Trade and Cooperation Agreement Explained, January 2021.
https://trade.ec.europa.eu/
doclib/docs/2021/january/
tradoc_159266.pdf
2. Rutter J, Thimont Jack. Managing the UK’s relationship with the European Union. Institute for Government. 4 February 2021.
https://www.instituteforgovernment.org.uk
/sites/default/files/publications/
managing-uk-relationship-eu.pdf
3.Charles Grant. Deadly coronavirus, domineering China and divided America: what the new geopolitics means for Europe. The new geopolitics: Annual Report 2020. Centre for European Reform 2021.
4. Catlow R. What does the UK-EU deal mean for science? Spring 2021 issue of Science in Parliament. The Journal of the Parliamentary and Scientific Committee – All-Party Parliamentary Group.
5. OECD. OECD Economic Outlook, December 2020. Volume 2020 Issue 2.
(All links last accessed February 2021)
COMMENTS ARE WELCOME






