Anomalies of the optic nerve are relatively rare, but account for a significant proportion of sight impairment in children and adults. The recognition of these anomalies by eye health professionals is important not only due to their potential impact on sight, but also because of their systemic associations. Here we review four of the most common optic nerve anomalies encountered in children.
Optic nerve hypoplasia
Optic nerve hypoplasia (ONH) is a common congenital anomaly of the optic nerve, representing a leading cause of visual impairment in children behind cortical vision impairment (CVI) in the UK and Europe [1]. Optic nerve hypoplasia includes a range of anatomical and clinical presentations, from hypoplasia of one or both optic nerves to associated extensive midline brain malformations, hypothalamic-pituitary dysfunction, and neurodevelopmental disorders such as autism spectrum disorder (ASD) [1,2]. The disorder is non-progressive and characterised histologically by a reduction in retinal ganglion cells and their axons, with relative sparing of the outer retinal layers [3].

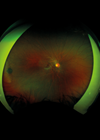
Figure 1: Bilateral Optos™ pseudocolour photographs of a four-year-old girl with left unilateral optic nerve hypoplasia. There is a characteristic ‘double ring’ sign with small calibre optic nerve. On the left, we demonstrate the measurement of ‘disc-disc to disc-macula ratio’ for determining possible hypoplasia. The horizontal diameter of the optic nerve is compared with the distance from the temporal edge of the disc to the macula.
Clinically, ONH may present as bilateral visual impairment and nystagmus in infancy or later as unilateral vision loss and strabismus in school-age children. Characteristic fundus findings, including a small pale disc (measured by horizontal disc-macula to disc-disc diameter, Figure 1) and the double-ring sign, support the diagnosis. A disc-macula to disc-disc ratio of three is considered suggestive of ONH, while a ratio of four or more meets criteria for ONH. It can be confirmed by ancillary investigations such as optical coherence tomography (OCT) and neuroimaging. However, the OCT findings of reduced disc size and loss of retinal nerve fibre and ganglion cell layers can be encountered in other diseases such as Leber’s hereditary optic neuropathy and dominant optic atrophy, so caution should be taken not to diagnose ONH in these progressive genetic optic neuropathies [1].
The pathogenesis of ONH is multifactorial, with both environmental and genetic contributors. Established environmental risk factors include young maternal age, primiparity and exposure to substances such as alcohol, recreational drugs or certain medications during pregnancy [1]. Although most cases are sporadic, mutations in key developmental genes for the optic nerves and midbrain – including HESX1, PAX6, SOX2 and OTX2 – may play a role in a minority of cases [3,4]. These genes encode transcription factors critical for early forebrain and ocular development. Notably, ONH is a core feature of septo-optic dysplasia, a condition defined by the presence of at least two of the triad: ONH, midline brain abnormalities (such as the absence of the septum pellucidum or corpus callosum), and hypopituitarism [4]. Importantly, children with ONH, regardless of MRI findings, remain at higher risk for evolving endocrinopathies and neurodevelopmental impairments, including ASD and intellectual disability [1,2].
Children with bilateral ONH can have severe sight impairment and should be referred for low vision support at diagnosis. In children with unilateral ONH, occlusion therapy is often not helpful – especially if the affected nerve is severely hypoplastic. Strabismus surgery can be undertaken for psychosocial reasons.

Figure 2: Image A, morning glory disc anomaly in a child, complicated by exudative retinal detachment. There is a typical pattern of spoke-like radiating blood vessels with posteriorly displaced and excavated optic nerve. A glial tuft is visible at 2–3 o’clock. In image B, a typical disc appearance is shown with rounded excavated disc, radial emanation of blood vessels from the disc and abnormal central glial tissue.

Figure 3: Images A and B: Optos™ widefield photographs of bilateral optic disc coloboma involving the disc and macula. In image C, Optos photograph of a coloboma involving the disc is shown. In image D of the same child, OCT through the nerve shows a deep excavation at the origin of the nerve, with thin and disorganised retina extending downward above the excavation from the coloboma.
Optic disc coloboma
Optic disc coloboma is a congenital anomaly with a prevalence of 8.9 per 100,000 children and is one of the most common optic nerve malformations behind ONH. It arises from incomplete closure of the embryonic fissure at weeks 6–7 of gestation [5]. This results in focal excavation involving the optic nerve head and contiguous chorioretinal coloboma [6]. Visual acuity is often reduced depending on the degree of foveal involvement. Concurrent lens or iris coloboma and microphthalmia may be observed. Some optic nerve coloboma may also be associated with a cyst, which can sometimes communicate with the subarachnoid space [5]. Amblyopia and refractive errors are also commonly seen.
Retinal complications such as serous retinal detachment, retinoschisis at the edge of the coloboma, progressive neuroretinal rim thinning, concurrent disc pit and choroidal neovascular membranes have been documented, particularly in extensive defects due to coloboma [5].
OCT can demonstrate retinochoroidal-scleral excavation, and OCT angiography shows the absence of the peripapillary microvascular network [7,8]. Herniated retinal tissue may be visible in the colobomatous area [7].
There are multiple possible systemic associations, including CHARGE syndrome (coloboma, heart defects, atresia of the nasal choanae, retardation of growth / development, genitourinary and ear anomalies) and Aicardi syndrome [5,6]. Aicardi syndrome is a disorder of unknown cause in individuals with two X chromosomes, where the primary features are central nervous system anomalies (CNS) such as agenesis of the corpus callosum, refractory seizures, severe developmental delay and chorioretinal lacunae. These lacunae can be focused around the optic disc, and individuals can also have other ocular anomalies such as optic disc, retinochoroidal and iris coloboma, retinal detachment and microphthalmia.
Patients with disc coloboma therefore require multidisciplinary involvement to investigate and manage systemic malformations. Other systemic associations include renal coloboma syndrome or other PAX2-associated disorders, CNS abnormalities, and PAX6 mutations [7,9,10].
Management involves amblyopia treatment, monocular precautions if unilateral (i.e. use of protective eyewear for contact sports or high-risk activities), and referral to a paediatrician to evaluate for systemic involvement [5]. Refractive error is more common in children with optic disc coloboma, and so annual cycloplegic refraction should be undertaken in this cohort [5,6]. Due to the risk of retinal detachment, regular fundus examination every 6–12 months is advised. Vitreoretinal input is valuable to determine whether prophylactic barrier laser may be of benefit, although this is not a universal practice. Identifying the foveal location with a chorioretinal coloboma is crucial for determining visual prognosis [5].
Morning glory syndrome
Morning glory anomaly or morning glory syndrome (MGS) is a congenital anomaly characterised by a posteriorly displaced optic nerve with a funnel-like excavation, radiating blood vessels with a spoke-like pattern, and residual glial tissue [6,7]. Vision can range from normal to very poor, and it is most commonly unilateral, although bilateral cases can occur. The incidence is 3.6 per 100,000 [7].
Significant systemic associations should be considered, including carotid circulation anomalies (Moyamoya disease), frontonasal dysplasia, basal encephalocoele, midline brain malformations and Aicardi syndrome [6]. Posterior fossa brain malformations, hemangiomas, arterial lesions, cardiac anomalies, eye anomalies, sternal clefting (PHACES) syndrome is another systemic disorder which includes MGS [11]. Upon identification of MGS, patients should be referred to a paediatrician to rule out systemic associations, and an MRI of the brain should be performed to look for malformations and vascular anomalies [6,11].
Retinal detachment occurs in up to 30% of patients with MGS and is most commonly exudative, although tractional and rhegmatogenous detachments may also occur [6].

Figure 4: Right optic disc pit with subretinal fluid and disruption of the outer retinal layers seen on OCT. There is minimal intraretinal fluid seen here, which is less typical of optic disc pit maculopathy.
Optic disc pit
Optic disc pits (ODPs) occur most commonly as congenital anomalies, with an estimated prevalence of 1–2 per 10,000 individuals and no gender predilection [12]. They are typically unilateral, with bilateral involvement seen in 10–15% of cases [6]. Optic disc pits most frequently occur in the inferotemporal quadrant of the optic disc and present clinically as greyish oval or round depressions. The underlying pathogenesis is thought to involve a defect in the lamina cribrosa, possibly due to abnormal closure of the embryonic fissure [13]. Histopathologically, the lesion represents herniation of dysplastic retinal tissue into the subarachnoid space [12].
Although many cases remain asymptomatic, 25–75% of patients develop optic disc pit maculopathy typically between the second and fourth decades of life [13]. This may present with blurred vision, micropsia or metamorphopsia and can result in significant visual impairment due to macular schisis and / or neurosensory retinal detachment.
In ODP maculopathy, OCT reveals intraretinal fluid (commonly within the outer nuclear and inner nuclear layers), neurosensory retinal detachment, and direct communication between the optic pit and the schisis cavity or subretinal space [7,12]. These findings support the hypothesis of fluid originating either from the vitreous cavity or the subarachnoid space via the pit [12]. Fluorescein angiography is generally non-contributory but may be useful in excluding mimics such as central serous chorioretinopathy.
Management strategies range from observation – particularly in mild cases or spontaneous resolution (seen in up to 25%) – to surgical intervention [12]. The most commonly employed procedure is pars plana vitrectomy, often combined with gas tamponade, internal limiting membrane peeling and flap, or laser photocoagulation [12,13]. Emerging adjuncts include human amniotic membrane and autologous fibrin, with varying levels of anatomical and functional success depending on the chronicity and extent of maculopathy [12].
TAKE HOME MESSAGES
-
Optic nerve hypoplasia is a leading cause of childhood visual impairment; is non-progressive and can be isolated, or associated with midline brain abnormalities, hypopituitarism, and neurodevelopmental disorders. It requires neuroimaging and endocrine assessment.
-
Morning glory syndrome presents with a characteristic optic disc appearance and is associated with serious systemic anomalies (e.g. PHACE, Moyamoya); retinal detachment is a common complication. Paediatric review and neuro-imaging are a mainstay of the initial work up due to the high rate of neurological and syndromic associations.
-
Optic disc coloboma results from failed embryonic fissure closure, can coexist with systemic syndromes (e.g. CHARGE, PAX2 disorders), and carries a risk of retinal complications – necessitating regular monitoring.
-
Optic disc pit is usually unilateral and may cause maculopathy in young adults; OCT is key in diagnosis, and management ranges from observation to vitrectomy.
-
Systemic evaluation and multidisciplinary care are crucial across all optic nerve anomalies due to frequent syndromic and neurodevelopmental associations.
References
1. Ryabets-Lienhard A, Stewart C, Brochert M, Geffner MC. The Optic Nerve Hypoplasia Spectrum: Review of the Literature and Clinical Guidelines. Adv Pediatr 2016;63(1):127–46.
2. Mann A, Aghababaie A, Kalitsi J, et al. Neurodevelopmental impairments in children with septo-optic dysplasia spectrum conditions: a systematic review. Mol Autism 2023;14(1):26.
3. Chen C-A, Yin J, Lewis RA, Schaaf CP. Genetic causes of optic nerve hypoplasia. J Med Genet 2017;54(7):441–9.
4. Ganau M, Huet S, Syrmos N, et al. Neuro-Ophthalmological Manifestations Of Septo-Optic Dysplasia: Current Perspectives. Eye Brain 2019;11:37–47.
5. Manta AS, Olsson M, Ek U, et al. Optic Disc Coloboma in children - prevalence, clinical characteristics and associated morbidity. Acta Ophthalmol 2019;97(5):478–85.
6. Jeng-Miller KW, Cestari DM, Gaier ED. Congenital anomalies of the optic disc: insights from optical coherence tomography imaging. Curr Opin Ophthalmol 2017;28(6):579–86.
7. Cennamo G, Rinaldi M, Concilio M, Costagliola C. Congenital Optic Disc Anomalies: Insights from Multimodal Imaging. J Clin Med 2024;13(5):1509.
8. Cennamo G, Rossi C, Ruggiero P, et al. Study of the Radial Peripapillary Capillary Network in Congenital Optic Disc Anomalies With Optical Coherence Tomography Angiography. Am J Ophthalmol 2017;176:1–8.
9. Okumura T, Furuichi K, Higashide T, et al. Association of PAX2 and Other Gene Mutations with the Clinical Manifestations of Renal Coloboma Syndrome. PLoS One 2015;10(11):e0142843.
10. Azuma N, Yamaguchi Y, Handa H, et al. Mutations of the PAX6 gene detected in patients with a variety of optic-nerve malformations. Am J Hum Genet 2003;72(6):1565–70.
11. Rotter A, Samorano LP, Rivitti-Machado MC, et al. PHACE syndrome: clinical manifestations, diagnostic criteria, and management. An Bras Dermatol 2018;93(3):405–11.
12. Esmaeil A, Ali A, Almutairi S, et al. Congenital optic disc pits and optic disc pit maculopathy: a review. Front Ophthalmol 2023;3:1222979.
13. Wan R, Chang A. Optic disc pit maculopathy: a review of diagnosis and treatment. Clin Exp Optom 2019;102(6):495–502.
Declaration of competing interests: None declared.