Meibomian gland dysfunction (MGD) is ubiquitous. Ocular surface inflammation and irritation are prevalent in most ophthalmology clinics: corneal, cataract, glaucoma, oculoplastic, paediatric, vitreo-retinal, medical retina and refractive surgery. These patients also represent roughly one third of those attending for emergency eye care.
Over the last decade the Tear Film and Ocular Surface Society (TFOS www.tearfilm.org) has published evidence-based reports from international workshops on dry eye [1], meibomian gland dysfunction [2] and contact lens discomfort. A second Dry Eye Workshop is in progress at the moment and the report is expected in September 2016.
Lacrimal gland failure is rare and Sjögren syndrome accounts for less than 2% of dry eye [3]. Other causes of lacrimal gland dysfunction such as sarcoid infiltration and graft-versus host disease are also rare. The vast majority of dry eye patients have MGD causing symptoms and signs of evaporative dry eye. If a patient can ‘cry’ and produce tears for whatever reason, lacrimal gland function is confirmed. We can assess dry eye and the impact of different therapeutic interventions by measuring a number of parameters.
Comprehensive objective assessment of MGD associated evaporative dry eye may include; evaluation of the glands themselves using infrared meibography, gland expressibility, the quality of meibomian secretions, quantifying gland ‘drop out’, tear film osmolarity, thickness of the lipid layer of the pre-corneal tear film, tear film stability and break-up time, height of the tear film meniscus which is a proxy for tear film volume, conjunctival hyperaemia and vascularity and ocular surface staining with the vital dyes – lissamine green, fluorescein and rose bengal. There are a plethora of dry eye questionnaires some validated, others not. The ocular surface disease index (OSDI) is a validated dry eye questionnaire widely used in dry eye research and is a useful tool for comparing different interventions and cross cohort comparison [4]. The OSDI is an objective measure of subjective symptoms, which is a Patient Reported Outcome Measure (PROM). PROMs are particularly important when assessing the impact of a therapeutic intervention on the patients’ quality of life because they measure whether patients report symptomatic improvement or not [5].
A useful summary of the evidence based management of MGD can be downloaded as a free PDF from the TFOS website:
http://www.tearfilm.org/
pdfs/TFOS_Mgd_Report_Overview.pdf
These strategies can be incorporated directly into practice with immediate benefit for both patients and eye care professionals. These interventions can be remembered, in sequence, using the mnemonic BOWELS (See Figure 1). B is for Blinking and is a prompt to address environmental issues, which promote evaporation of the pre-corneal tear film. O is for Omega 3 dietary intake, which may need to be increased. W is for Warming of the eyelids, which needs to be safe, effective and acceptable for patients to comply. E is for Expression of the meibomian secretions. L is for both Lid hygiene and Lubricants including artificial tears and gels. S is for Systemic treatments, Steroid drops and Scripts – treatment only available on prescription.
B – Blinking
O – Omega 3
W – Warming
E – Expression
L – Lubricants and Lid hygiene
S – Systemic. Steroid drops. Scripts
Figure 1: B.O.W.E.L.S.
Blinking
The normal temperature of the anterior human eye is about 35ºC with the eyelid margins between 34º and 35ºC. Assuming an ambient atmospheric temperature of 20ºC the human eye functions as a spheroidal radiant heat source with a thin liquid film on its surface with a 15ºC temperature difference at the tear film surface. Evaporation of the watery component of the tear film, driven by the temperature difference, is exacerbated by low humidity environments and airflow over the ocular surface from wind, draughts, air conditioning, car heaters, fans, etc. Tear film evaporation is retarded by the superficial tear film lipid-layer.
When the lipid-layer breaks up prematurely there is rapid evaporation of tear-film water, which results in increased concentration of tear film solutes with simultaneous increasing tear osmolarity. This hyper-osmolarity is particularly dramatic at the apex of the tear meniscus where it is thinnest. Because hyper-osmolarity causes cellular insult by a different mechanism from alkali, thermal, electrical or mechanical injury, the pattern of damage is different. The lid margins are particularly vulnerable to hyper-osmolar insult when meibomian secretions are inadequate and the normal epithelial protective barrier function of the meibomian oil is lost. Just as the globe itself is a heat source, so too is the lid margin. The meibomian glands are at normal body temperature whereas the orifices themselves interface with an ambient environmental temperature 15ºC cooler. When the inter-blink time (the time between each blink) exceeds the pre-corneal tear film break up time (TFBUT), evaporation increases.
The normal non-invasive tear film lipid layer break up time is 10 seconds or longer. TFBUT less than 10 seconds is deemed to be abnormal. Close attentive gaze profoundly reduces the blink rate. Blink frequency is influenced by the caudate nucleus within the basal ganglia and this is apparent in Parkinson’s disease where the blink frequency is diminished. Reduction in blink frequency is a normal part of the near response and is neuro-anatomically hard-wired the same way as convergence, accommodation and miosis – one cannot ‘choose’ to blink more often when in close attentive gaze because it is beyond one’s control. Many aspects of modern life involve prolonged periods of close attentive gaze, with computer work and smartphone use being widespread. It is helpful to give patients a ‘blinking strategy’, which will depend on their pursuits, but encourages them to refresh their pre-corneal tear film frequently.
Omega
Omega 3 fatty acids (Ω3) have been shown to have a direct anti-inflammatory effect on macrophages by down-regulating both the NFkB and JnK pro-inflammatory pathways. The GPR-120 cell membrane receptor is specific for Ω3 fatty acids and the mechanism of action has been elucidated and elegantly described in work by Jerry Olefsky’s team at the San Diego Department of Medicine [6]. Adipocytes also express the GPR-120 receptor and there is good reason to treat diabetic patients (non insulin dependent diabetes mellitus (NIDDM) and pre-diabetes) with dietary Ω3 supplements. The European and British regulatory frameworks do not permit routine prescription of dietary supplements so these have to be purchased by the patient. They can easily be obtained from optometry practices or online. There are now many different commercial preparations of Ω3 available with different formulations. The combination of docosahexaenoic acid (DHA) eicosapentaenoic acid (EPA) and gamma linolenic acid (GLA) - (a ‘good’ omega 6) has been shown to be effective [7]. Blister packed capsules are preferable as omega 3 fatty acids oxidise in air.
As a general rule if the Ω3 capsules give you ‘fishy burps’ they have undergone oxidation and are less desirable. Absorption and hence bioavailability of omega 3 is enhanced when ingested at the end of the main meal on a full stomach. The Dry Eye Assessment and Management Study (DREAM) organised by Penny Asbell and Maureen Maguire, is a US multicentre randomised double blind study of omega 3 supplementation in dry eye. It is in progress and the results may clarify the role of omega 3 fatty acids in dry eye treatment [8].
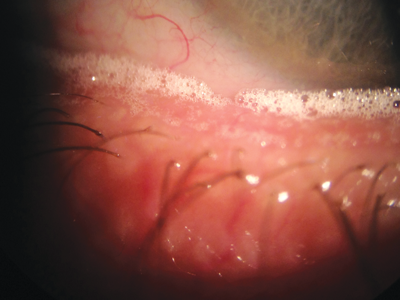
Figure 2: ‘Champagne bubbles’ along the lower lid margin characteristic of MGD.
Warming
Thermal injury can occur with temperatures above 45ºC. Effective warming of the eyelids induces a phase change in the inspissated secretions plugging the meibomian orifices liquefying the solid grease and reducing its viscosity. This allows egress of the meibomian oil onto the lid margin and the ocular surface, ‘lubricating the blink’ while replenishing and maintaining a normal lipid layer for the pre-corneal tear film. For a warm compress (WC) to be safe and effective it must warm the lids and tarsal plates above body temperature for several minutes without causing thermal injury. Compliance improves when the warm compress is easy and pleasant to use. Caroline Blackie and colleagues attempted to determine an optimal method of hot wet face cloth warm compress application but concluded “customised, labour-intensive WC procedure is necessary to optimise treating meibomian gland dysfunction and obstruction using WCs [9].”
In a randomised, single masked, contralateral clinical trial of 25 patients with confirmed MGD-related evaporative dry eye, the MGDRx EyeBag® delivered a statistically significant improvement in all eleven different dry eye parameters while corneal topography and visual acuity were unaffected. There was a significant improvement in meibomian gland dropout, which was unexpected and had been thought to be an untreatable late consequence of MGD.
All patients maintained higher ocular comfort after six months although the benefit was greater in those who continued to use the Eyebag® one to eight times a month. The authors conclude “… EyeBag® is a safe and effective device for the treatment of MGD-related evaporative dry eye. Subjective benefit lasts at least six months, aided by occasional retreatment [10].” Microbiological studies conducted on EyeBags® experimentally inoculated with Staph aureus, Strep pyogenes and Pseudomonas aeruginosa demonstrate that “…heating (in a microwave oven in accordance with the instructions for use) produces a significant reduction in bacterial load to levels typically measured on the eyelid margins of healthy people [11].”
Several other eyelid warming devices are now available commercially but the EyeBag® is the first and presently, the only such device available on prescription anywhere in the world. The EyeBag® may be prescribed for NHS patients throughout the UK from 1st October 2015.
Expression
In order to unblock the meibomian glands it is necessary to express the inspissated secretions. This can be carried out at the slit-lamp using a cotton bud, the tip of a minim or an instrument specifically designed for this purpose of which there are several. However, for long-term management and to release clinic time, it is preferable that the patient ‘self-expresses’ his or her own meibomian glands regularly. Patients’ manual dexterity and hand eye coordination must be taken into account when massage techniques are being described.
Although patients can understand how to express using the index finger pad, many prefer and better comprehend the ‘Tired Toddler Technique’ using a knuckle and firmly closed eyes. It is important that the patients are instructed to do this lid massage immediately they remove their warm compress when the meibomian secretions are most amenable to expression.
Lid hygiene and lubricants
Lid hygiene is a major topic in itself, especially the vexed question of the role of baby shampoo. Crusty eyelash deposits, collarettes in particular, are strongly associated with colonisation of the lash follicles by the arthropod mite, Demodex folliculorum, which are best observed with a slit-lamp using 40x magnification.
Many different lid-cleaning products can be purchased direct from optometrists or online, some purporting to eradicate demodex. Evans and Guy at Cardiff University School of Optometry, conducted a randomised, double blind, contralateral eye study of 19 patients comparing Blephasol (Théa Pharmaceuticals) with Johnson’s Baby Shampoo 10:1 dilution (Johnson & Johnson). After lid-scrub with dilute baby shampoo, tear film break-up time was reduced significantly and conjunctival, limbal and lid margin hyperaemia increased significantly, while alterations in tear film stability and hyperaemia were insignificant with Blephasol lid / lash debridement. They conclude: “Dilute baby shampoo appears to have a greater adverse impact on short-term tear film stability and ocular surface physiology compared to the other product. These results add support for the use of a specially formulated lid hygiene product compared to dilute baby shampoo [12].”
Lubricant eye drops and gels commonly known as artificial tears give symptomatic relief. There are 77 different such preparations listed with their NHS Tariff prices, as ‘eye products’ in Part IXA- Appliances of the August 2015 UK NHS Electronic Drug Tariff [13]. The NHS expenditure on prescription lubricant eyedrops was circa £65 million in 2014. Lubricant eye preparations fall into several broad groups based on the main ingredient: carmellose (carboxymethylcellulose), hydroxypopyl guar, hypromellose, polyvinyl alcohol, sodium hyaluronate, soybean oil, carbomer and paraffin based ointments. Preservative free preparations are much more expensive. The lubricant you prescribe is often dictated by what is available in your local trust formulary, which in turn is usually dictated by price. Some of the preparations have evidence of efficacy published in peer-reviewed journals and some have been shown to improve the ocular surface but their main function is lubrication and relief of discomfort.
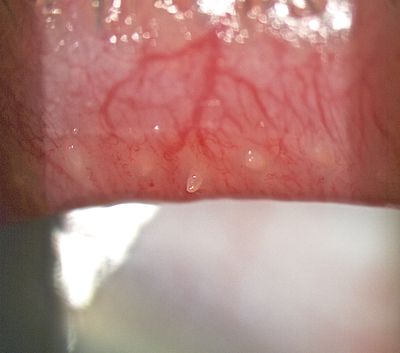
Figure 3: Solid meibomian secretion plugging the meibomian
orifice and causing foreign body sensation on blinking.
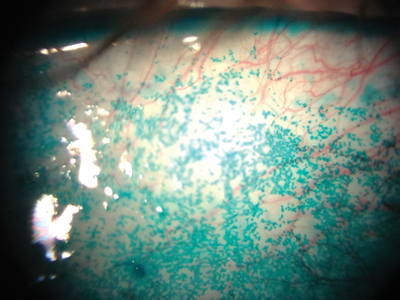
Figure 4: Lissamine green staining of the conjunctiva with typical speckled pattern in dry eye.
Systemic, steroids drops and scripts
Mohsen Kashkouli and colleagues in Tehran studied 110 patients with MGD comparing five days systemic azithromycin with 28 days systemic doxycycline and concluded: “Five-day oral azithromycin is recommended for its better effect on improving the signs, better overall clinical response and shorter duration of treatment.” They gave 500mg of azithromycin on day one, followed by 250mgs once a day for a further four days [14]. This regime may also be useful in the treatment of rosacea both with and without ocular involvement. Steroid eye drops are sometimes required for severe MGD but the usual caveats apply. Loteprednol has been used prior to the commencement of topical cyclosporine to accelerate symptomatic relief.
Several topical cyclosporine preparations are presently available with more in the pipeline. There is also a veterinary preparation of cyclosporine eye ointment often used to treat severe dry eye in dogs – notably west highland terriers, which are prone to a Sjögren like eye condition. This veterinary cyclosporine (used off label – and off species in fact) is often effective for severe and refractory inflammatory ocular surface disease including rosacea keratitis and maintaining remission in peripheral ulcerative keratitis (PUK), Moorens ulcer, rheumatoid corneal melt, sclerokeratitis and some cases of scleritis.
There are several new pharmacologically active eye drops, which show promise for the ocular surface: Anakinra, an IL-1 receptor antagonist formulated as a 2.5% eye drop solution is safe and significantly reduced symptoms and epitheliopathy in dry eye patients [15]. Diquafasol tetrasodium 3%, a P2Y2 purinergic receptor agonist, improved both signs and symptoms in dry eye patients with Sjögren syndrome [16]. Lifitegrast, a small molecule integrin antagonist made up as a 5% ophthalmic solution, significantly reduced corneal fluorescein and conjunctival lissamine green staining and improved symptoms of ocular discomfort and eye dryness over an 84 day period when compared with placebo in a study of 588 patients with dry eye [17]. Rebamipide ophthalmic suspension adopts yet another therapeutic approach; originally developed to treat gastric ulcers by increasing production of gastric mucin, rebamipide has a similar effect on the ocular surface, increasing conjunctival mucin production, which anchors the glycocalyx improving the integrity of the tear film [18].
Symtoms and signs mismatch in dry eye
It is perplexing to observe many patients where compelling dry eye symptoms do not appear to correlate with less florid signs of dry eye. Recent publications suggest that patients with disproportionate dry eye symptoms may have chronic neuropathic ocular pain with allodynia and hyperalgesia [19] and often have contemporaneous fibromyalgia (recently rebadged by the rheumatologists as Chronic Widespread Pain Syndrome (CWPS)), irritable bowel syndrome, pelvic pain syndrome and depression [20]. Randomised controlled trials of treatment for CWPS support the use of the serotonin norepinephrine re-uptake inhibitor duloxetine [21], which is otherwise prescribed in general practice as an antidepressant. Where dry eye symptoms exceed signs and the patient has concomitant CWPS (with or without depression) there may be scope for an individual therapeutic trial of duloxetine 60mg once a day to treat the CWPS, with anticipation of fortuitous improvement in the dry eye symptoms.
The ocular surface is a complex arrangement of different tissues, structures and secretions. Many diverse specialties and areas of expertise and research converge here and there is genuine cause for optimism about future management options for the wide range of diseases and conditions, which cause ocular surface misery for so many people.
References
1. 2007 Report of the International Dry Eye Workshop (DEWS). Ocul Surf 2007;5(2).
2. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction. Invest Ophthalmol Vis Sci 2011;52(4):1922-9.
3. Thomas E, Hay EM, Hajeer A, Silman AJ. Sjögren’s syndrome: a community-based study of prevalence and impact. Br J Rheumatol 1998;37:1069-76.
4. Dougherty BE, Nichols JJ, Nichols KK. Rasch analysis of the Ocular Surface Disease Index (OSDI). Invest Ophthalmol Vis Sci 2011;52(12):8630-5.
5. Grubbs JR, Tolleson-Rhinehart S, Huynh K, Davis RM. A review of quality of life measures in dry eye questionaires. Cornea 2014;33(2):215-8.
6. Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142(5):687-98.
7. Sheppard JD Jr, Singh R, McClellan AJ, et al. Long-term supplementation with n-6 and n-3 PUFAs improves moderate-to-severe keratoconjunctivitis sicca: a randomized double-blind clinical trial. Cornea 2013;32(10):1297-304.
8. Dry Eye Assessment and Management Study (DREAM). Clinical trials.gov. Identifier NCT02128763.
https://clinicaltrials.gov/ct2/show/NCT02128763
Last accessed December 2015.
9. Blackie CA, Solomon JD, Greiner JV, et al. Inner eyelid surface temperature as a function of warm compress methodology. Optom Vis Sci 2008;85(8):675-83.
10. Bilkhu PS, Naroo SA, Wolffsohn JS. Randomised masked clinical trial of the MGDRx eyebag for the treatment of meibomian gland dysfunction-related evaporative dry eye. Br J Ophthalmol 2014;98:1707-11.
11. Bilkhu P, Wolffsohn J, Naroo S, et al. Effects of therapeutic and sanitisation heating on the microbiology of warm compress treatment. Abstracts of the BCLA clinical conference. Liverpool 29-31 May 2015. Number 44.
http://www.bcla.org.uk/resources/events/
bcla-clinical-conference-a-exhibition/1942
Last accessed December 2015.
12. Evans K, Guy L. Comparing the short-term effect of two different lid hygiene products on ocular signs and symptoms. Abstracts of the BCLA Clinical Conference. Liverpool 29-31 May 2015. Number 39.
http://www.bcla.org.uk/resources/events/
bcla-clinical-conference-a-exhibition/1942
Last accessed December 2015.
13. NHS Electronic Drug Tariff.
http://www.drugtariff.nhsbsa.nhs.uk/
#/00280039-DC/DC00280030/Home
Last accessed December 2015.
14. Kashkouli MB, Fazel AJ, Kiavash V, et al. Oral azithromycin versus doxycycline in meibomian gland dysfunction: a randomised double-masked open-label clinical trial. Br J Ophthalmol 2015;99:199-204.
15. Amparo F, Dastjerdi MH, Okanobo A, et al. Topical Interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. JAMA Ophthalmology 2013;131(6):715-23.
16. Yokoi N, Sonomura Y, Kato H, et al. Three percent diquafosol ophthalmic solution as an additional therapy to existing artificial tears with steroids for dry-eye patients with Sjögren’s syndrome. Eye (Lond) 2015;29(9):1204-12.
17. Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology 2014;121(2):475-83.
18. Kashima T, Itakura H, Akiyama H, Kishi S. Rebamipide ophthalmic suspension for the treatment of dry eye syndrome: a critical appraisal. Clin Ophthalmol 2014;8:1003-10.
19. Galor A, Levitt RC, Felix ER, et al. Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye 2015;29(3):301-12.
20. Vehof J, Kozareva D, Hysi P, Hammond CJ. Prevalence and risk factors of dry eye disease in a British female cohort. Br J Ophthalmol 2014;98:1712-7.
21. Roskell NS, Beard SM, Zhao Y, Le TK. A meta-analysis of pain response in the treatment of fibromyalgia. Pain Pract 2011;11(6):516-27.
Further reading
-
www.tearfilm.org/dewsreport/
-
http://iovs.arvojournals.org/Issues.aspx?issueID=932970 - issueid=932970
-
www.drugtariff.nhsbsa.nhs.uk/ - /00232686-FA/FA00232184/Part IXA-Appliances
-
www.bcla.org.uk/resources/events/bcla-clinical-conference-a-exhibition/1942
-
https://clinicaltrials.gov/ct2/show/NCT02128763
-
www.ncbi.nlm.nih.gov/pubmedhealth/PMH0041438/
-
www.tearfilm.org
-
www.eyebags.com
Declaration of Competing Interests
The author has a significant financial interest in the EyeBag®. He is Managing Director of The EyeBag Company Ltd and is the majority shareholder. He also owns the intellectual property – Patent numbers GB2421687B & EU 05818615.6
COMMENTS ARE WELCOME