The global increase in Acanthamoeba keratitis infections has emphasised the inefficiencies of current treatment and preventative methods, here researchers from the West of Scotland detail a promising new series of compounds that may stem the tide.
News headlines detailing horror stories of contact lens wearers losing their sight to the once uncommon eye-eating amoeba, Acanthamoeba, are becoming increasingly frequent. Acanthamoeba keratitis (AK) infections are on the rise, but why is this the case and what is being done to combat the upsurge of this devastating pathogen?
Here we provide an overview of recent studies on the pathogen, focusing mainly on preventative strategies developed to help reduce transmission of Acanthamoeba from the environment to the cornea through improved contact lens sterilisation. AK infections are increasing globally and the need for effective combative methods has never been greater, as such, we also discuss a promising new series of compounds that may provide an answer to the global rise of this life-changing infection.
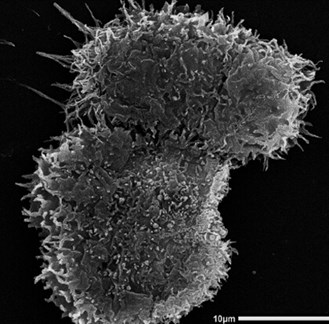
Figure 1: Scanning Electron Microscopy monograph of the trophozoites of Acanthamoeba castellanii.
The trophozoite is approximately 25um.
Acanthamoeba, an opportunistic parasite of humans
Acanthamoeba are free-living, unicellular organisms, ubiquitous in both natural and man-made environments. Interconversion between the two life-stages of the amoeba allows survival in almost all environments and as such, they can be found in soil, aquatic environments and more importantly in water systems, such as domestic taps, cooling units and swimming pools [1]. The trophozoite (Figure 1) is the active life-stage, existing in optimal conditions and survives ordinarily by feeding on bacteria in the environment. The associated symptoms of AK stem from the activity of these trophozoites within the eye, which they access through improperly sterilised contact lenses.

Figure 2: Acanthamoeba can enter the eye by attaching to contact lenses (1), the depleted immune activity allows binding to the epithelial layer (2) and the amoeba can begin to feed on the epithelial cells (3). Micro-abrasions on the epithelial layer provides an opportunity for the amoeba to access the Bowman’s membrane and stroma of the patient (4). Drug pressure can instigate encystation of the parasite (5) which can then begin reinfection upon removal of this pressure (6).
Beneath the contact lens, the amoeba are able to evade the immune response of the eye and can attach to, and feed on, the epithelial cells. They can then enter the corneal layers through microscopic abrasions in the epithelial layer, allowing them to cause further damage to the Bowman’s membrane and eventually the stroma of the patient [2] (Figure 2). Acanthamoeba can cause significant damage throughout the cornea and additional damage can be facilitated by the recruitment of immune cells to the eye and the associated inflammatory response [2].
When the trophozoite encounters stress (e.g. lack of nutrients, fluctuations in temperature, pH and moisture) it can transform into a dormant cyst stage to allow survival in harsher environments. From a clinical perspective, drugs and other inhibitors that may be found in current contact lens solutions provide stressful environments for the trophozoites, prompting the defensive response of the parasite and initiating encystation [3].
“Existing contact lens solutions are highly effective in the elimination of bacterial and fungal pathogens, but less so, Acanthamoeba”
The cyst is covered in a strong cellulose wall that acts as a barrier to the external environment and while encysted cellular metabolism decreases, at which point the amoeba can survive for several decades if necessary [4]. As a result, the majority of currently available treatments are ineffective against the cyst form and it is this stage that provides the most significant barrier in both prevention and treatment. Removal of the stressor or cessation of drug treatment in AK can allow the cyst to revert to the active trophozoite stage and very often leads to re-emergence of the infection [5]. Unfortunately, disease resurgence in AK is common even after extreme treatment methods such as corneal transplants and the physical and mental impact this can have on those afflicted is significant.
Epidemiology
Acanthamoeba can come into contact with the contact lens in a variety of ways. Bathing, swimming or even rinsing lenses with tap water can provide an opportunity for the cell to attach to lenses and initiate infection. Unfortunately, many of the readily available contact lens cleaning solutions fail to kill Acanthamoeba and as such, the overnight incubation that is so effective for most microorganisms is unable to eliminate the risk of infection. Consequently, these inefficiencies, coupled with the immune privilege of the eye, particularly while wearing contact lenses, means that 95% of AK cases in developed countries are associated with contact lens use [6].
Currently, there are approximately three million AK patients, although this number is likely an underestimation given that it is not mandatory to report the disease [7]. Incidence rates of AK also vary greatly from country to country. The UK has around 15 times more cases than the United States and even within the UK, regional differences exist between Scotland and England.
“Existing contact lens solutions are highly effective in the elimination of bacterial and fungal pathogens, but less so, Acanthamoeba”
The reasons for these differences is a culmination of factors, for example water storage tanks widely used in the UK, but less so in the United States, provide an ideal environment for free-living amoeba to thrive and ultimately increase the likelihood of coming in to contact with humans [8]. In all regions, AK infections are increasing [9], this is due in part to the improved awareness of AK and earlier and more effective diagnosis that distinguishes Acanthamoeba keratitis from other symptomatically similar keratitis infections [10].
Curative treatment strategies
Drugs such chlorohexidine, brolene, neomycin, miltefosine and polyhexamethylene biguanide (PHMB) are effective individually and in combination against trophozoites but less so against cysts [11]. Steroids are also given to reduce corneal inflammation, but only when the number of Acanthamoeba has reduced significantly, as steroids, such as dexamethasome, can promote Acanthamoeba growth and can reduce immune function within the eye [12]. Interestingly, chlorhexidine and PHMB combined are cysticidal, but have to be administered over prolonged periods (more than six months) to ensure the complete eradication of the amoeba. Unfortunately, damage to eye also progresses and blindness may ensue, before treatment completion. The slow pharmacokinetics of some anti-acanthamoebic compounds and their presence in plasma at suboptimal concentrations induces cyst formation, making treatment ineffective. Upon encystation of Acanthamoeba, symptoms will subside and drug pressure will be eased, this allows the encysted cells to revert to the active trophozoites and cause resurgence of the disease.
Preventative strategies
Existing contact lens solutions are highly effective in the elimination of bacterial and fungal pathogens, but less so, Acanthamoeba. The use of hydrogen peroxide containing contact lens solutions is commonplace. These solutions are available as one- or two-step solutions and depending on the type, offer different results against Acanthamoeba. Not only this, but the efficacy of these compounds also varies depending on the amount of cells or specific strain of Acanthamoeba. Studies using the one-step method prove to be largely ineffective against the cysts of Acanthamoeba, likely due to the rapid neutralisation of the hydrogen peroxide [13].
As such, a solution that fails to act upon cysts as well as trophozoites cannot fully prevent infection. The two-step solutions, however, are effective against both stages and would be the preferred solution for combatting the parasite [13]. This method is not without its drawbacks though. Alternatively, solutions not relying on hydrogen peroxide tend to use a multi-compound approach to disinfect lenses. Compounds such as polyaminopropyl biguanide, PHMB, polyqutarenium and ethylenediaminetetraacetic acid (EDTA) are used in combination, their efficacy against Acanthamoeba though varies. While many of these compounds are effective against trophozoites, they fail to effectively eradicate cysts, and in many cases can induce encystation if they fail to kill trophozoites at a sufficient speed [14]. Some of these combinations at certain concentrations can have cysticidal activity, unfortunately these same combinations are detrimental to corneal cells and are unusable in solutions. It is a recurring theme then, that while we have several compounds that can kill the trophozoite stage, we lack an effective compound that can kill cysts without causing damage to the eye itself. The surge in recent cases of AK and the lack of effective anti-Acanthamoeba compounds has emphasised the need for further research aimed at tackling this problem.
The ideal preventative strategy
There is a growing need for an effective preventive strategy to eliminate the risk of Acanthamoeba contaminating contact lenses and infecting the eye. Three elements need to be considered in the assessment of an effective compound: i) it has to possess fast pharmacokinetics that do not induce encystation ii) it has to be active against the cysts and iii) ideally it would work in combination with other anti-microbial agents for an improved contact lens solution for contact lens hygiene and cleansing. To date, compounds such as these are limited. Recently though, our team has been successful in the identification of a series of compounds that are effective against both Acanthamoeba trophozoites and cysts and remain hopeful that they may offer a new method of combatting the global rise of these parasites.

Figure 3: Structures of QACs and APCs with anti-amoebic activities.
Advancement in AK treatment
Quaternary ammonium compounds (QAC) and alkylphosphocholines (APC) demonstrate a promising and an effective avenue for new treatments (Figure 3). APCs and QACs are broadly classified as surfactants, and are comprised of i) a charged head which can have an overall positive, negative or neutral charge; ii) a tail comprised of saturated or unsaturated alkyl carbon chains, and iii) a carbon linker between the head and the tail. APCs have shown wide-ranging efficacy against several pathogens and have even been shown to be effective in the treatment of Acanthamoeba trophozoites when combined with other compounds [15,16]. Similarly, QACs possess antimicrobial [17] properties and certain variations of these QACs are currently used in contact lens solutions (e.g. Polyquart). As is often the case in Acanthamoeba targeting compounds, the currently available APCs and QACs fail to effectively act on the cysts. We have identified several QAC and APC compounds that are highly effective individually, and in combination, that target both the infectious and defensive stages of Acanthamoeba. Screening a variety of structurally different QACs and APCs, we have successfully demonstrated that slight alterations in net-charge of the molecule or the length of the alkyl-carbon tail can have a huge impact on the efficacy of the compound against Acanthamoeba (Figure 3).
QACs possess a net positive charge (cationic) while APCs have both a negative and a positive charge (zwitterionic). This change from zwitterionic to cationic results in a significant change in the compounds ability to kill the amoeba, with the cationic compounds being able to cause death at much lower concentrations than is observed in the zwitterionic counterparts. In addition to this, by increasing the length of the alkyl-carbon tail of both APCs and QACs the compounds become more potent against Acanthamoeba.
To understand why this is the case we must first understand how these molecules behave in solution. Surfactants such as QACs and APCs can exist in aqueous solutions individually until a certain concentration is exceeded, beyond this point the individual compounds aggregate to form spherical structures known as micelles. The point in which these compounds begin to form micelles is termed the critical micelle concentration (CMC), a concentration that decreases as the tail length of the experimental compounds increases (Figure 4).

Figure 4: Critical micelle formation of QACs is influenced by tail length (a). Short-tail QACs are unable to form micelles on the cell membrane and instead are broken down to produce energy (b) while long-tail QACs form micelles on the cell membrane resulting in intracellular leakage and death (c).
We have shown that by forming micelles on the membrane of Acanthamoeba these compounds can cause leakage of intracellular components from the cell, which ultimately results in death (Figure 4) [18]. Increasing the tail length of these compounds decreases the amount of the compound needed to form these micelles and thus results in a more efficient compound. It is interesting though to note that slight changes in the structural composition of these molecules can have major impacts on their efficacy, a point demonstrated by the contrasting effects of QAC12 relative to longer tailed QACs. While QACs possessing an alkyl-carbon tail with more than 12 carbons can exert toxicity, the 12-carboned QAC has the opposite effect. The inability of the compound to form micelles on the amoeba membrane results in its utilisation as an energy substrate, breaking down the compound and resulting in a rapid increase in cell growth (Figure 4) [18]. Despite this, QAC12 might also prove useful in the development of an anti-Acanthamoeba formulation as it not only acts as an energy source, but it is able to delay the encystation of the amoeba for as much as 96 hours. Combined therapies using QAC12 to delay encystation alongside slow acting compounds may inhibit the ability of the amoeba to enter the defensive cyst stage long enough for the second compound to exert its effect [18].
Combating resistance to QACs
Plasticity of the genome and metabolism of parasites means that the development of antimicrobial resistance is a frequent occurrence. Acanthamoeba are no different in this respect and there are several documented cases of resistance to commonly used drugs arising from misuse [19,20]. The ability to utilise short-tail QACs suggests that resistance to longer tailed variants may be possible if mismanaged. For treatment to remain effective, and their continuous use or integrity maintained, there is a need to understand the route used to develop resistance. In anticipation of this, we have developed a strain of Acanthamoeba highly resistant to QACs and are using this as a model to understand the molecular, biochemical and cellular approaches undertaken to develop this resistance. Through this research we have not only shown that resistance is possible but shown how to identify and combat this resistance should it arise in the environment. Recognising important biomarkers in resistant amoeba and developing alternative combined treatment methods that use these resistance mechanisms to our advantage, we hope to remain one-step ahead in this clinical arms race.
Conclusion
The recent explosion in AK cases has highlighted how drastically underprepared we are to deal with the problem. Here we have listed a variety of compounds used in the treatment or prevention of this incredibly resilient parasite, none of which have been developed with the treatment or prevention of the parasite in mind. Our breakthrough in the finding of a series of effective anti-Acanthamoeba compounds is fortified by not only the multiplicity of the compounds and their ability to work in combination with other compounds, but in the additional work that has been compiled into potential resistance mechanisms and in the mechanism of action of these effective compounds specifically with the prevention of Acanthamoeba keratitis in mind. Integration of these compounds into contact lens solutions is currently underway and further understanding of how the compounds interact with other components of the solution is being investigated. Our hope is that by taking an Acanthamoeba specific approach we can help prevent the further rise of this debilitating disease. In finally being able to combat the defensive cyst stage of the parasite, we may see a drastic reduction in those afflicted by Acanthamoeba keratitis and the increasingly common headlines eluding to contact lens seeking, eye-eating amoeba may eventually become a thing of the past.
TAKE HOME MESSAGE
-
Global incidence rates of Acanthamoeba keratitis (AK) are on the rise.
-
The adaptability of the Acanthamoeba parasite makes treatment extremely difficult and disease resurgence is common.
-
Current treatment methods are ineffective, either failing to effectively kill the defensive cyst stage or promoting encystation of the active trophozoites.
-
AK is predominately associated with contact lens wear due to the poor activity of current contact lens solutions against Acanthamoeba.
-
Quaternary ammonium compounds (QACs) and alkylphosphocholines (APCs) present promising alternatives for adding to contact lens solutions in an attempt to halt the global rise of AK.
-
Structural alterations to QACs and APCs can have profound effects on their efficacy and understanding the mechanisms behind this can allow for the development of more effective combined therapies, identification of resistance biomarkers and methods for tackling resistance should it arise.
References
1. Khan NA. Acanthamoeba: Biology and increasing importance in human health. FEMS Microbiol Rev 2006;30:564-95.
2. Lorenzo-Morales J, Martín-Navarro CM, López-Arencibia A, et al. Acanthamoeba keratitis: An emerging disease gathering importance worldwide? Trends Parasitol 2013;29:181-7.
3. Campbell SJ, Ingram PR, Roberts CW, Henriquez FL. Induced encystment improves resistance to preservation and storage of Acanthamoeba castellanii. Parasitology 2008;135:1401-5.
4. Sriram R, Shoff M, Booton G, et al. Survival of Acanthamoeba cysts after desiccation for more than 20 years. J Clin Microbiol 2008;46:4045-8.
5. Mazur T, Hadas E, Iwanicka I. The duration of the cyst stage and the viability and virulence of Acanthamoeba isolates. Trop Med Parasitol 1995;46:106-8.
6. Ibrahim YW, Boase DL, Cree IA. How could contact lens wearers be at risk of Acanthamoeba infection? A review. J Optom 2009;2:60-6.
7. Schlossberg D: Clinical Infectious Disease. Cambridge University Press; 2008.
8. Kilvington S, Gray T, Dart J, et al. Acanthamoeba Keratitis: The role of domestic tap water contamination in the United Kingdom. Invest Ophthalmol Vis Sci 2004;45:165-9.
9. Carnt N, Hoffman JJ, Verma S, et al. Acanthamoeba keratitis: Confirmation of the UK outbreak and a prospective case-control study identifying contributing risk factors. Br J Ophthalmol 2018;102:1621-8.
10. Alexander CL, Coyne M, Jones B, Anijeet D. Acanthamoeba keratitis: Improving the scottish diagnostic service for the rapid molecular detection of Acanthamoeba species. J Med Microbiol 2015;64:682-7.
11. Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015;22:10.
12. McClellan K, Howard K, Niederkorn JY, Alizadeh H. Effect of steroids on Acanthamoeba cysts and trophozoites. Investig Ophthalmol Vis Sci 2001;42:2885-93.
13. Hiti K, Walochnik J, Faschinger C, et al. One- and two-step hydrogen peroxide contact lens disinfection solutions against Acanthamoeba: How effective are they? Eye 2005;19:1301-5.
14. Fears AC, Metzinger RC, Killeen SZ, et al. Comparative in vitro effectiveness of a novel contact lens multipurpose solution on Acanthamoeba castellanii. J Ophthalmic Inflamm Infect 2018;8:19.
15. Walochnik J, Duchêne M, Seifert K, et al. Cytotoxic activities of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob Agents Chemother 2002;46:695-701.
16. Schuster FL, Guglielmo BJ, Visvesvara GS. In-vitro activity of miltefosine and Voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp., and Naegleria fowleri. J Eukaryot Microbiol 2006;53:121-6.
17. Carmona-Ribeiro AM, de Melo Carrasco LD. Cationic antimicrobial polymers and their assemblies. Int J Mol Sci 2013;14:9906-46.
18. Mooney R, Masala M, Martial T, et al. Alkyl-carbon chain length of two distinct compounds and derivatives are key determinants of their anti-Acanthamoeba activities. Sci Rep 2020;10:6420.
19. Hay J, Kirkness CM, Seal D V, Wright P. Drug resistance and Acanthamoeba Keratitis: The quest for alternative antiprotozoal chemotherapy. Eye 1994;8:555-63.
20. Iovieno A, Oechsler RA, Ledee DR, et al. Drug-resistant severe acanthamoeba keratitis caused by rare T5 acanthamoeba genotype. Eye Contact Lens 2010;36:183-4.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME








