The author highlights current debate, opinion and late breaking developments in the management of retinal diseases.
The American Academy of Ophthalmology’s 2019 Retina Subspecialty Day Meeting was held on 11-12 October, 2019 in San Francisco, USA. Established and emerging innovative approaches to the management of vitreoretinal diseases were presented, together with reviews of current and new clinical trial data for age-related macular degeneration (AMD), diabetic retinopathy (DR), hereditary retinal conditions and retinal vein occlusion (RVO).
Pro-Con debates
Management of epiretinal membranes
Dr Colin McCannel argued that early intervention for epiretinal membranes (ERMs) in eyes that still have good visual acuity (VA) is appropriate in many cases. Surgical intervention with vitrectomy and membrane peeling is highly effective in preventing progression of symptoms, improves VA in around two-thirds of patients and usually improves metamorphopsia. Retinal detachment risk is below 1%, endophthalmitis risk is less than 1/2000 and vision recovery time has reduced. Epiretinal membranes must be peeled before vision deteriorates significantly. Optimal indications proposed include VA 20/30 or worse (surgery definitely recommended if ≤20/40), any metamorphopsia and any loss of binocularity.
Eyes with idiopathic ERM and good VA are often stable and can be safely observed over an extended follow-up, with a low rate of progression, explained Dr Harry Flynn. Overall, 13% of eyes with newly diagnosed idiopathic ERMs who did not need surgical intervention progressed to needing surgery at seven years, according to results of a recent study [1]. Visual acuity improvements with surgery for ERMs are often modest, with residual metamorphopsia. Ophthalmologists were encouraged to consider treatment burden and impact on patients’ daily activities, not just the appearance of the optical coherence tomography (OCT) scan. In patients with phakic ERM, cataract surgery is often a good first step.
Anti-VEGF therapy is the best treatment for PDR
The beneficial effects of anti-vascular endothelial growth factor (anti-VEGF) therapy combined with few complications make it the preferred treatment for proliferative diabetic retinopathy (PDR), argued Dr Jeffrey Gross. Data from the Diabetic Retinopathy Clinical Research network (DRCR.net) Protocol S and CLARITY studies demonstrated that anti-VEGF therapy is either non-inferior or superior to panretinal photocoagulation (PRP) for the treatment of PDR [2,3]. While PRP remains an effective, proven treatment for PDR, it is associated with significant side-effects such as reduced visual fields, increased macula oedema and decreased night vision. Five-year outcomes for PDR show development of retinal detachment in 18% of PRP eyes versus 7% of eyes treated with ranibizumab (Lucentis®, Novartis) [4]. Moreover, PRP is not generally a ‘one shot and done’ procedure, as additional laser treatment is often required.
For PDR without diabetic macular oedema (DMO), PRP (single shot, not pattern scan) is a better approach than anti-VEGF monotherapy, countered Dr Dean Eliott. Panretinal photocoagulation is highly durable, more cost-effective and provides better outcomes when patients are lost to follow-up.
Non-proliferative diabetic retinopathy (severe NPDR) should be routinely treated with anti-VEGF therapy
Intervention with anti-VEGF therapy for high-risk non-proliferative PDR (NPDR) can improve retinopathy and reverse vision loss, noted Dr Diana Do [5-7]. Patients with moderately severe to severe NPDR at baseline benefit the most from anti-VEGF therapy. One-year results of the PANORAMA study of aflibercept (Eylea®, Bayer / Regeneron) for NPDR without DMO found that treatment reduced the risk of developing proliferative disease and centre-involved DMO (CI-DMO) by approximately 74% compared with sham injection [7]. The number needed to treat to prevent one vision threatening complication or CI-DMO through week 52 was three.
Dr Yannek Leiderman argued that anti-VEGF treatment is best used selectively rather than routinely in eyes with severe NPDR. Final results of DRCR.net Protocol W study are awaited. This study will provide evidence of safety and efficacy of prompt anti-VEGF versus observation in eyes presenting with severe NPDR and no CI-DMO for prevention of vision-threatening outcomes.
OCT angiography is essential for clinical practice
Optical coherence tomography angiography (OCT-A) has become an essential new tool in the armamentarium of the retinal specialist, argued Dr Nadia Khalida Waheed. In clinical practice, OCT-A provides a non-invasive alternative to dye-based angiography to visualise blood vessels and assist in the diagnosis of choroidal neovascularisation (CNV) and for follow-up of patients with DR and other retinal vascular disease. It keeps vision safe and is faster and more reliable than invasive testing.
Is it time to convert to OCT-A today? Not at this time, according to Dr Richard Kaiser. Currently, clinically relevant uses for OCT-A in retina practice are limited. Image acquisition artefacts can lead to incorrect interpretations of images and the technology provides no information on vascular permeability [8].
When treating nAMD, macular fluid should not be tolerated
Undertreatment of neovascular age-related macular degeneration (nAMD) leads to vision loss and maintaining a dry retina correlates with vision gain, asserted Dr David Brown. The PIER study found that those with active OCT lesions (i.e. leakage) at months five and eight lost vision from baseline at month 24, while those with inactive OCT lesions achieved vision gains [9]. Tolerating fluid with pro re nata (PRN, or ‘persistent retinal neglect’) anti-VEGF treatment regimens leads to vision loss. In the Comparison of Age-related Macular Degeneration Treatments Trials (CATT) study, eyes with residual intraretinal fluid (IRF) had worse mean VA than those without at all time points through one year [10]. Moreover, persistent retinal fluid requires more frequent treatment [11].
The FLUID study showed that if retinal fluid is tolerated, then final vision is poor: mean change in best corrected visual acuity (BCVA) from baseline to month 24 was 3.0 Early Treatment Diabetic Retinopathy Study (ETDRS) letters in the intensive treatment group (resolve all subretinal fluid [SRF] completely) vs. 2.6 letters in the relaxed group (tolerating some SRF) [12]. Multiple other treat-and-extend studies of anti-VEGF therapy for nAMD show substantially greater vision improvements than those achieved in the FLUID study. Up to 40% of participants had IRF at the end of the FLUID study [12].
Dr Joan Miller agreed that presence of IRF correlates with poorer visual outcomes but argued that some macular fluid may be tolerated. Recent interesting studies have investigated the relationship between vision outcomes and persistent SRF with active anti-VEGF treatment for nAMD. In the HARBOR study, those with residual SRF achieved the best vision outcomes through 24 months [13]. There was no difference in early visual function outcomes between eyes with or without SRF in an analysis of VIEW 1 and VIEW 2 clinical trial data [14]. Both HARBOR data and analysis of combined VIEW data indicate that residual IRF is associated with lower vision gains and poorer visual outcomes, replicating findings seen in other studies including CATT and IVAN. Not all retinal fluid is the same: SRF may be observed, for example, persistent SRF with active anti-VEGF therapy or for subthreshold CNV with good vision. Intraretinal fluid on the other hand indicates CNV activity and needs to be treated, while degenerative cysts should be observed.
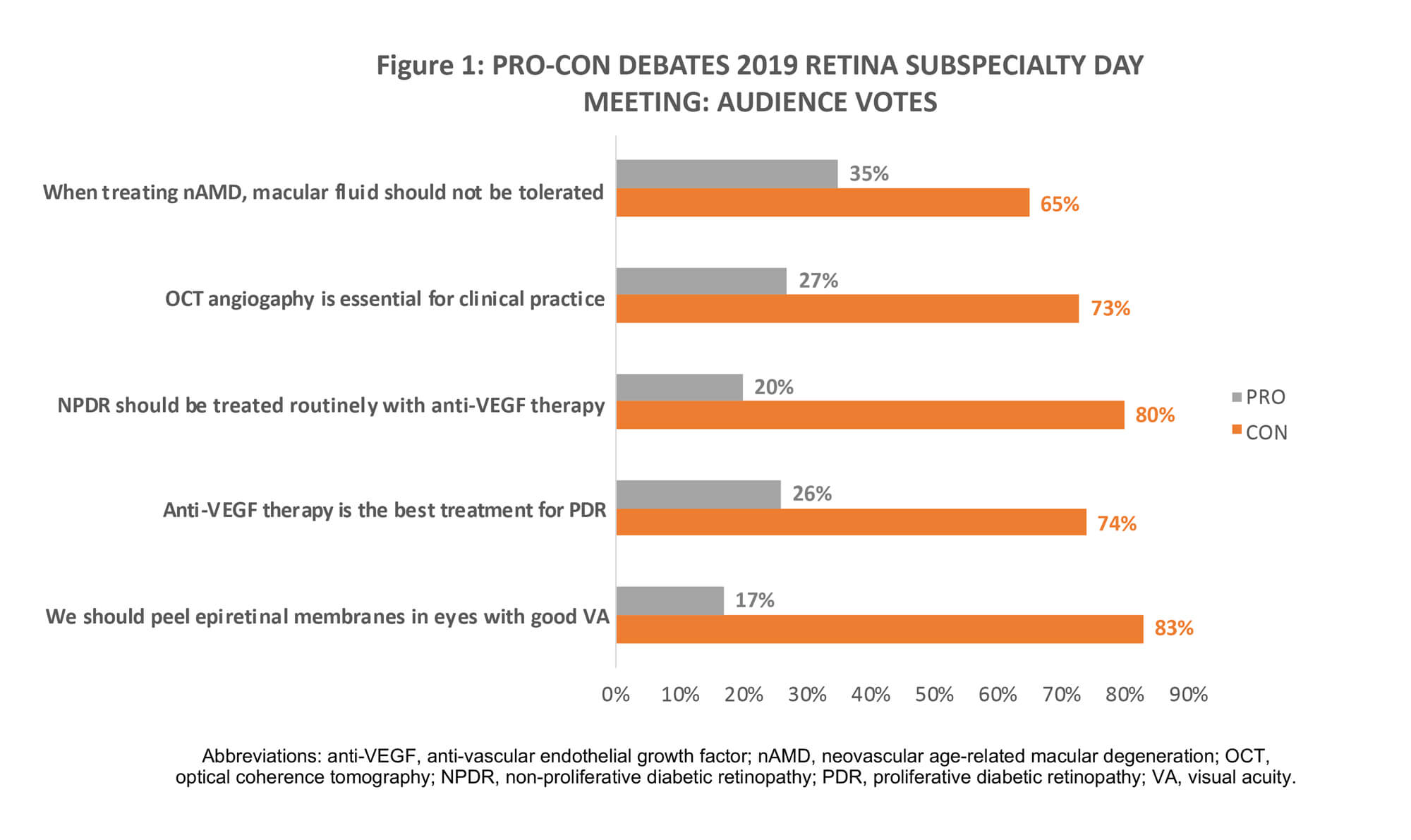
Figure 1 summarises audience votes following the respective pro-con debates. While the ‘Cons’ swept the session, presentations show there is room for debate.
What’s new in the management of neovascular AMD
The functional impact of fluctuations in retinal thickness
Current evidence suggests that VA outcomes in nAMD are not affected by fluctuations in retinal thickness during anti-VEGF treatment. A meta-analysis of the IVAN and CATT studies tested the hypothesis that fluctuations in retinal thickness are associated with poor functional outcomes (analysis population, n=1,711). Over two years, eyes in higher quartiles of SD (spread around the mean) foveal centre point thickness exhibited increasingly less VA improvement and a higher frequency of fibrosis. Treatment durability and fluid resolution are important for preservation of both function and morphology, noted Professor Usha Chakravarthy.
Predictors of visual outcomes in nAMD
What anatomic factors predict visual outcome in patients being treated for nAMD? Five-year results from the CATT study found that worse vision was associated with subretinal hyper-reflective material (SRHM) / scar / fibrosis, foveal atrophy and IRF, while foveal SRF and subretinal pigment epithelium (sub-RPE) fluid were associated with better vision [15].
Dr Srinivas Sadda presented results of a multicentre, retrospective study of 204 eyes of 177 patients with nAMD treated with anti-VEGF therapy and followed for at least five years. The presence and thickness of the pigment epithelial detachment (PED) under the fovea was an important predictor of long-term vision in patients being treated for nAMD. Results showed that thicker PED and thinner SRHM (especially under the fovea) were associated with better vision. Prospective longitudinal trials are needed to further evaluate optimal morphologic endpoints for successful anti-VEGF therapy in patients with CNV.
Brolucizumab for typical nAMD and polypoidal choroidal vasculopathy: 96-week results from HAWK
Intravitreal VEGF inhibitor brolucizumab (Beovu®, Novartis) was recently approved by the US Food and Drug Administration (FDA) for the treatment of nAMD [16]. The recommended regimen is treatment every 8 to 12 weeks after the first three monthly doses [16]. Regulatory approval was based on findings from the phase 3 HAWK and HARRIER clinical trials, which demonstrated non-inferiority of brolucizumab 6mg every 12 weeks / every eight weeks (q12w/q8w) versus aflibercept 2mg q8w after loading in mean change in BCVA at week 48 for treatment naïve nAMD (Figures 2 and 3) [17]. Through week 96, the proportion of patients who were maintained on brolucizumab q12w dosing ranged between 39% and 45% [16].
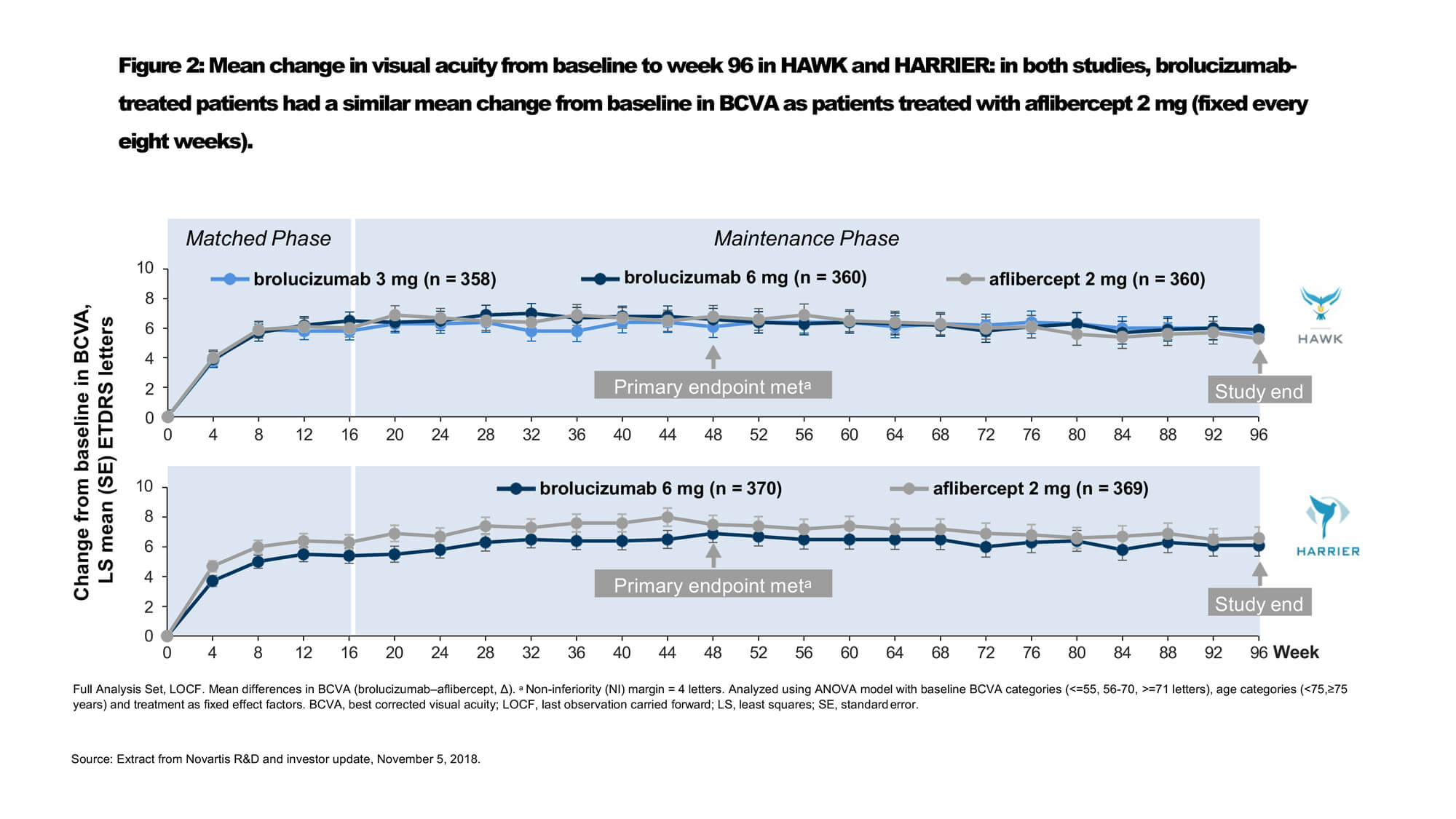
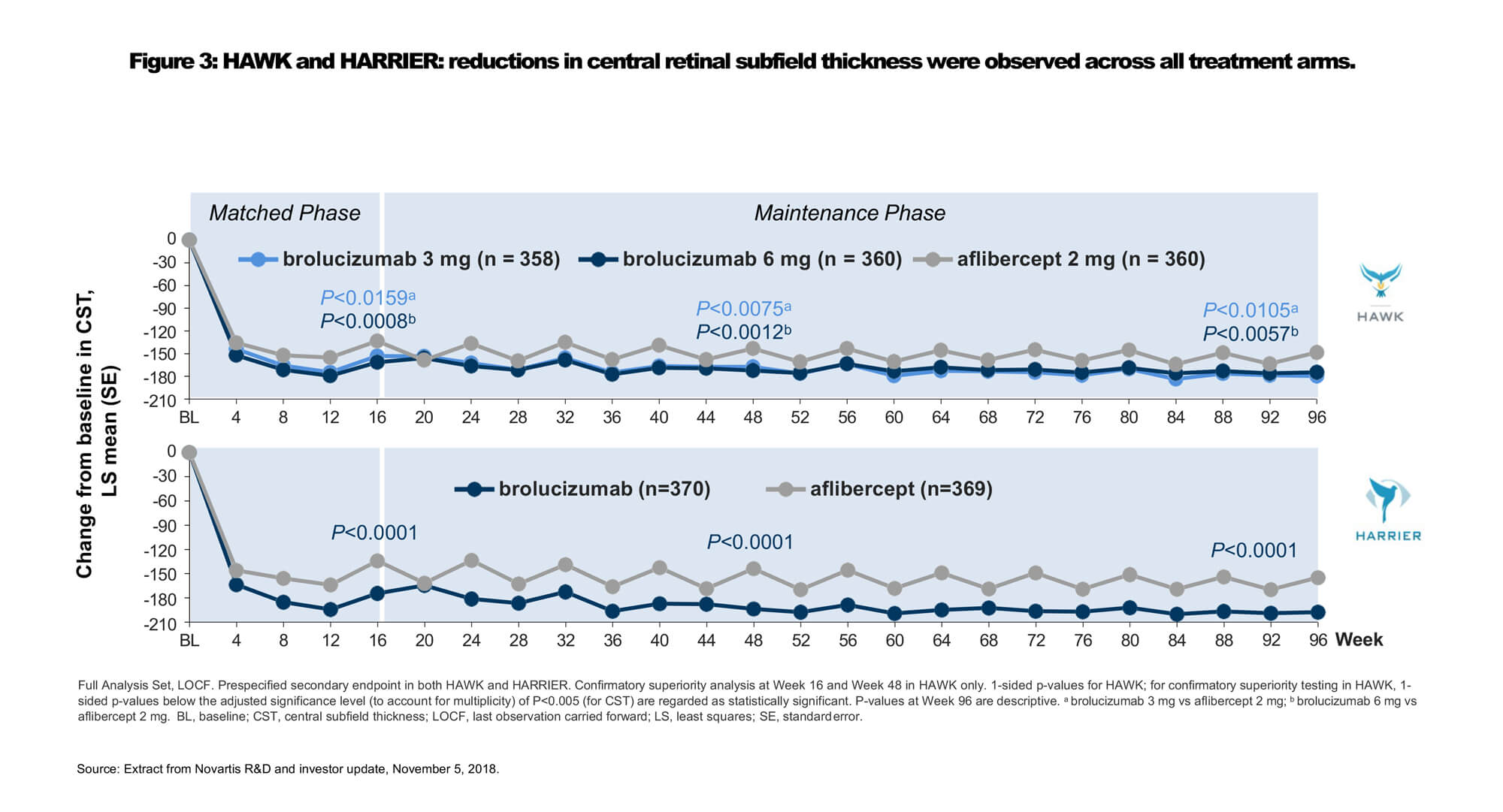
Dr Glenn Jaffe discussed 96-week results of a subanalysis of the HAWK study evaluating outcomes in Japanese participants with polypoidal choroidal vasculopathy (PCV). Robust BCVA gains were observed across all treatment arms, confirming the efficacy of anti-VEGF monotherapy for both typical nAMD and PCV patients. The mean BCVA change from baseline at weeks 48 and 96 was 10.4 and 11.4 letters, respectively, in the brolucizumab 6mg group (n=39), and 11.6 and 11.1 letters in the aflibercept group (n=30). Two-thirds (68%) of Japanese PCV patients treated with brolucizumab 6mg were maintained on quarterly dosing immediately following the loading phase to week 96.
Late breaking developments
Two-year results from phase 3 studies of abicipar vs. ranibizumab for nAMD
Dr Rahul Khurana reported two-year pooled results from the CEDAR and SEQUOIA phase 3 studies of investigational abicipar (Allergan) compared to ranibizumab for nAMD. Abicipar treatment effect (stable vision, loss of <15 letters from baseline) at week 52 was maintained in the second year with fewer injections. Mean change in BCVA from baseline at week 104 was +7.8, +6.1 and +8.5 in abicipar q8w, abicipar q12w and monthly ranibizumab groups, respectively. The pooled rate of new cases of intraocular inflammation in year two (weeks 52 to 104) for abicipar-treated patients was 1.9% (0.8% abicipar q8w and 2.3% abicipar q12w) compared with 1.0% in the ranibizumab monthly arm.
End of study results from the LADDER phase 2 trial
The Port Delivery System (PDS) with ranibizumab (Roche) has the potential to reduce the treatment burden for nAMD through continuous delivery of ranibizumab. Dr Carl Regillo presented LADDER phase 2 trial end of study results. Efficacy and safety outcomes were consistent with the primary analysis. In the PDS 100mg/mL arm, 80% of nAMD patients (previously responsive to anti-VEGF treatment) reached month six without requiring a refill and ~60% of patients went ≥12 months without requiring implant refill. In patients who required refills, the median time to first and second refills was consistent at 8.8 months. Across all treatment arms, PDS was well tolerated through the study duration (mean follow-up of 22.1 months).
Subretinal RGX-314 gene therapy for nAMD
RGX-314 (Regenxbio) is a recombinant adeno-associated virus (AAV) gene therapy vector carrying a coding sequence for a soluble anti-VEGF protein. The long-term, stable delivery of this therapeutic protein following a one-time gene therapy treatment for nAMD could potentially reduce the treatment burden of regular intravitreal injections while maintaining vision with a favourable benefit:risk profile. Dr Jeffrey Heier discussed findings from the RGX-314 phase 1/2a nAMD clinical trial, which has fully enrolled 42 patients in five dose cohorts. Enrolled participants were severe nAMD patients requiring frequent anti-VEGF injections. Subretinal RGX-314 was well tolerated with a dose dependent increase in ocular protein observed across cohorts. In cohort five (2.5x1011, n=12), the highest clinical response was observed with 75% of subjects injection-free at five to six months with stable to improved anatomic and visual outcomes (from baseline, central retinal thickness -68µm, BCVA +4 letters). Planned developments include an in-office delivery platform.
Intravitreal gene therapy with ADVM-022 for nAMD (OPTIC trial)
ADVM-022 (AAV.7m8-aflibercept) (Adverum Biotechnologies) is a recombinant, replication-deficient AAV gene therapy vector carrying a coding sequence for aflibercept. Dr Szilárd Kiss presented promising 24-week results of a phase I clinical trial (OPTIC) of intravitreal gene therapy with ADVM-022 for nAMD patients requiring frequent anti-VEGF injections to maintain their vision. Additional data from OPTIC Cohort 1 with a median follow-up of 34 weeks showed that a single ADVM-022 injection maintained vision, with consistent and sustained anatomical improvements on OCT, with no rescue injections required for any of the six patients. Inflammation was generally mild and manageable with steroid eye drops.
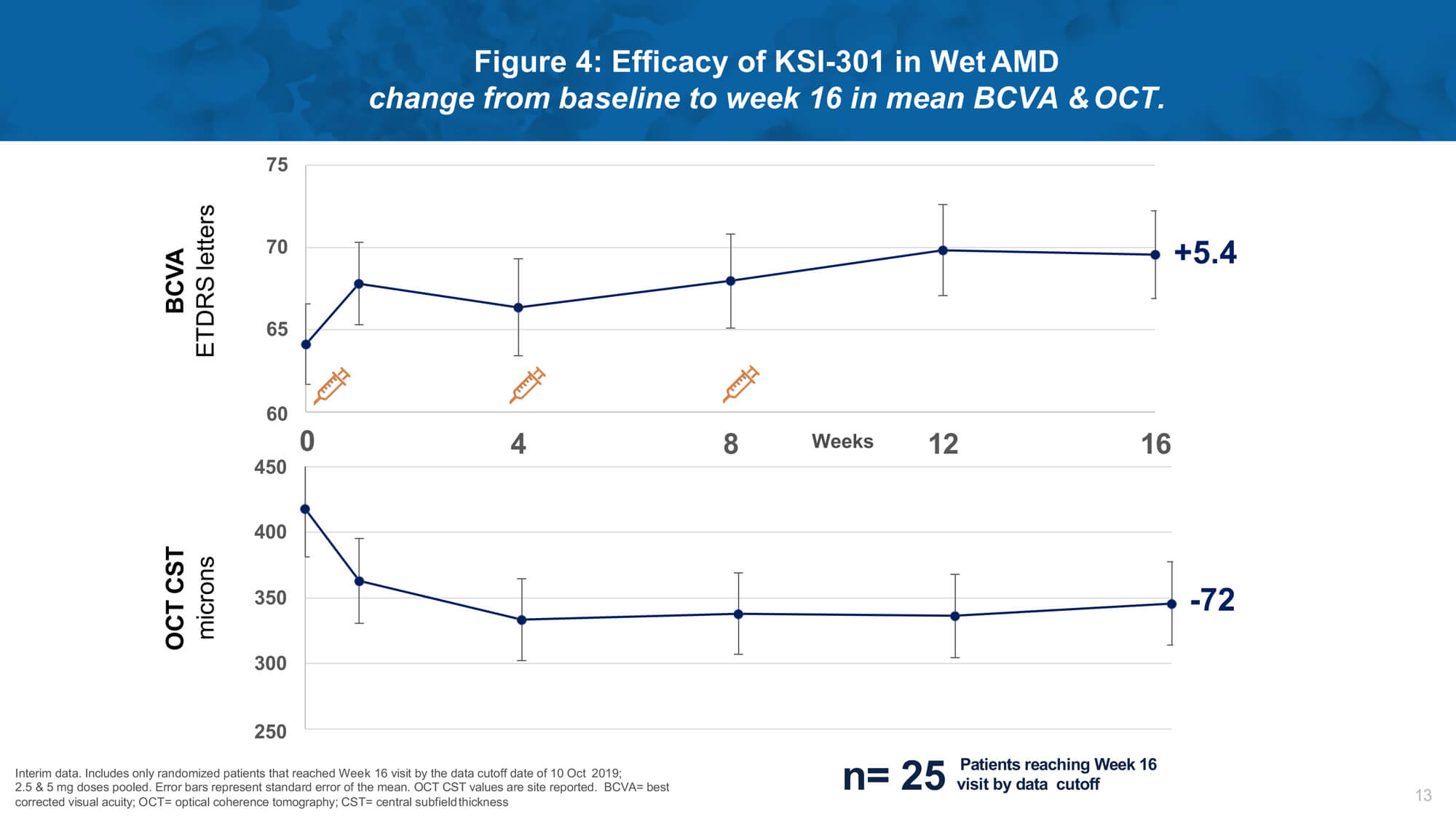
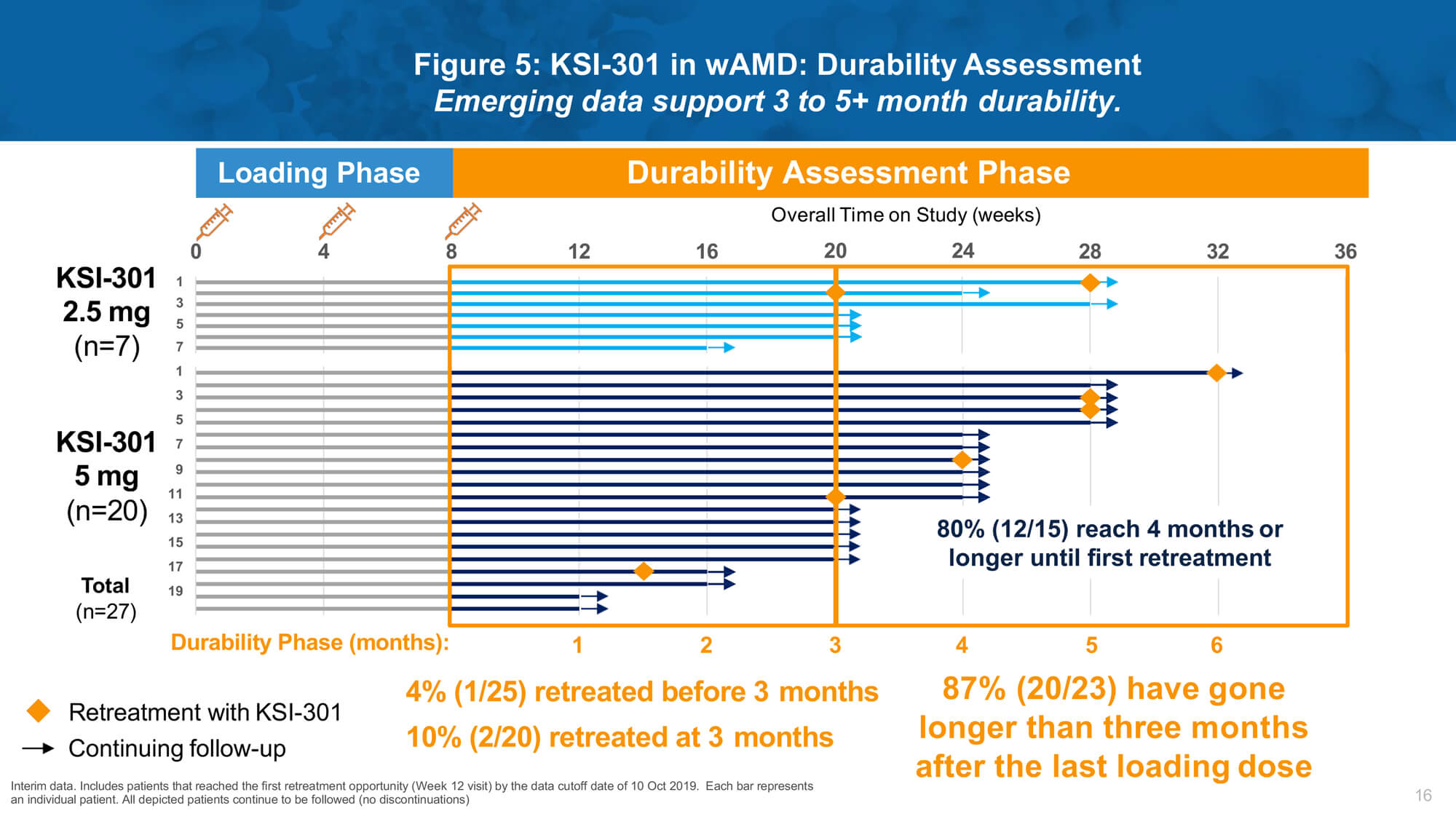
First-time results evaluating anti-VEGF antibody biopolymer conjugate
Investigational therapy KSI-301 (Kodiak Sciences) is an intravitreal anti-VEGF antibody biopolymer conjugate designed to block all VEGF-A isoforms. Dr Charles Wykoff presented results from a phase 1b study of KSI-301 demonstrating promising safety, efficacy and durability in patients with nAMD, DMO and RVO (Figures 4 and 5).
Remarkable biological durability was observed, with a majority of treated eyes extended to four months or beyond without requiring retreatment after three loading doses. A phase 2 study (DAZZLE) will evaluate KSI-301 5mg dosed every 12 to 20 weeks compared to aflibercept 2mg every eight weeks after three initial monthly loading doses in treatment-naïve nAMD patients.
Investigational treatment achieves improved vision in patients with dry AMD
Risuteganib (Luminate®, Allegro Ophthalmics) is a small synthetic peptide designed to regulate select integrin functions involved in the pathogenesis of dry AMD, with a retinal half-life of ~21 days. Impressive phase 2 study results were reported evaluating the efficacy of risuteganib intravitreal injection in patients with intermediate non-exudative AMD, with approximately half of patients seeing better following treatment (n=12/25). The primary endpoint of proportion of patients gaining ≥8 ETDRS letters was met (48% vs. 7% for sham, P=0.013). A good safety profile was observed. Additionally, ~1,200 injections have been given outside the study with an acceptable safety profile. A larger clinical trial is underway to confirm primary phase 2 findings.
The LEAVO study of anti-VEGF therapies for macular oedema due to central RVO
Top-line results of the LEAVO randomised non-inferiority study comparing the effectiveness of ranibizumab vs. aflibercept vs. bevacizumab for macular oedema due to central RVO were presented and discussed by Mr Philip Hykin [18]. For the management of macular oedema due to central RVO:
- bevacizumab was not non-inferior to ranibizumab at 100 weeks
- aflibercept was non-inferior to ranibizumab but not superior
- bevacizumab was not non-inferior to aflibercept at weeks 52 and 100
- at least eight weekly follow-up and prompt treatment in the second year maintained first-year VA gains.
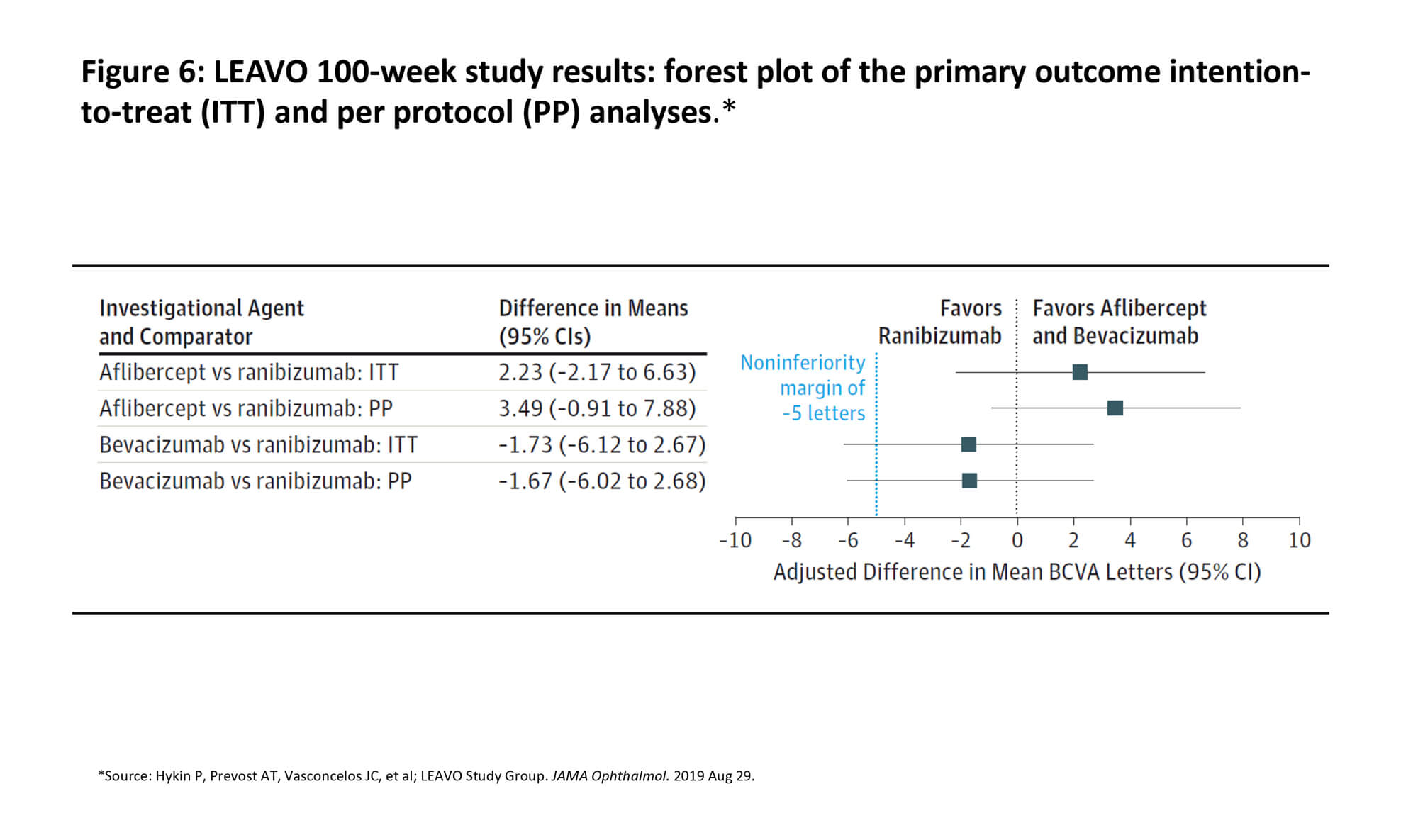
Repeated intravitreal anti-VEGF injections markedly improved and maintained BCVA among patients with macular oedema secondary to CRVO during follow-up of 100 weeks. Figure 6 presents a forest plot of the primary outcome intention-to-treat and per protocol analyses [18]. Treatment frequency through 100 weeks ranged from 9.8 to 11.9 injections. Fewer injections were required in the aflibercept arm vs. ranibizumab arm at weeks 52 and 100. Investigators concluded that, for RVO patients managed in the LEAVO study, there is low level of confidence for recommending bevacizumab as equivalent to ranibizumab and aflibercept over two years.
References
1. Chen X, Klein KA, Shah CP, Heier JS. Progression to surgery for patients with idiopathic epiretinal membranes and good vision. Ophthalmic Surg Lasers Imaging Retina 2018;49(10):S18-22.
2. Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 2015;314(20):2137-46.
3. Sivaprasad S, Prevost AT, Vasconcelos JC, et al; CLARITY Study Group. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet 2017;389(10085):2193-203.
4. Gross JG, Glassman AR, Liu D, et al; Diabetic Retinopathy Clinical Research Network. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol 2018;136(10):1138-48.
5. Wykoff CC, Eichenbaum DA, Roth DB, et al. Ranibizumab induces regression of diabetic retinopathy in most patients at high risk of progression to proliferative diabetic retinopathy. Ophthalmol Retina 2018;2(10):997-1009.
6. Mitchell P, McAllister I, Larsen M, et al. Evaluating the Impact of Intravitreal Aflibercept on Diabetic Retinopathy Progression in the VIVID-DME and VISTA-DME Studies. Ophthalmol Retina 2018;2(10):988-96.
7. Wykoff CC. PANORAMA Trial: 1-year results. Presentation at 2019 Angiogenesis, Exudation and Degeneration Symposium, February 9, 2019, Miami, FL, USA.
8. Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina 2015;35(11):2163-80.
9. Brown DM, Tuomi L, Shapiro H; Pier Study Group. Anatomical measures as predictors of visual outcomes in ranibizumab-treated eyes with neovascular age-related macular degeneration. Retina 2013;33(1):23-34.
10. Jaffe GJ, Martin DF, Toth CA, et al; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2013;120(9):1860-70.
11. Jaffe GJ, Kaiser PK, Thompson D, et al. Differential response to anti-VEGF regimens in age-related macular degeneration patients with early persistent retinal fluid. Ophthalmology 2016;123(9):1856-64.
12. Guymer RH, Markey CM, McAllister IL, et al; FLUID Investigators. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology 2019;126(5):723-34.
13. Sadda SR. Relationship between subretinal fluid (SRF) or intraretinal fluid (IRF) and vision outcomes in eyes with neovascular age-related macular degeneration (nAMD) treated with ranibizumab in the HARBOR trial. Presentation at The Retina Society 2019 Annual Meeting, September 11-15, 2019, London, UK.
14. Singh RP. Association between early visual function outcomes and anatomic dryness in neovascular age-related macular degeneration. Presentation at The Retina Society 2019 Annual Meeting, September 11-15, 2019, London, UK.
15. Jaffe GJ, Ying GS, Toth CA, et al; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Macular morphology and visual acuity in year five of the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology 2019;126(2):252-60.
16. BEOVU® (brolucizumab-dbll) injection [prescribing information]; East Hanover, NJ. Novartis: 2019.
17. Dugel PU, Koh A, Ogura Y, et al; HAWK and HARRIER Study Investigators. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2019 [Epub ahead of print].
18. Hykin P, Prevost AT, Vasconcelos JC, et al; LEAVO Study Group. Clinical effectiveness of intravitreal therapy with ranibizumab vs aflibercept vs bevacizumab for macular edema secondary to central retinal vein occlusion: a randomized clinical trial. JAMA Ophthalmol 2019 [Epub ahead of print].
COMMENTS ARE WELCOME






