The authors report on 35 consecutive new cases of isolated fourth cranial nerve palsy seen over a period of six months in one neuro-ophthalmology clinic in Southeast Asia, with emphasis on their aetiology and management.
We report on 35 patients with newly diagnosed isolated fourth cranial nerve (CN) palsies seen over a six month period between March and October 2014 in one of our neuro-ophthalmology consultant’s (JFC) clinics at the Singapore National Eye Centre (SNEC).
In the 2002-2004 survey of neuro-ophthalmic diseases in Singapore, derived from its specialist neuro-ophthalmology clinics in the four major hospitals, a total of 627 new patients were seen [1]. One hundred and eighty-six (29.6%) had disorders of motility, of these, 58 (31.1%) had third CN palsies, 47 (25.2%) fourth palsies and 81 (43.5%) involved the sixth nerve. In the age group 40-69 years, 64.5% of the ocular motor palsies were of presumed ischaemic origin associated with diabetes mellitus (DM) in 42.5%, hypertension (HTN) in 40%, and hyperlipidemia (HL) in 27% [1]. With regard to the fourth CN palsies in the same age group 65.7% had diabetes mellitus, 48.6% hyperlipidemia and 30% hypertension.
A presumed microvascular aetiology is attributed to the condition in those patients where clinical testing, absence of other neurological signs and neuro-imaging do not reveal an alternative cause and where spontaneous resolution of the paresis occurs [2].
Non-ischaemic fourth nerve palsies are most often due to head trauma, while others can be associated with a compressive lesion, a carotid cavernous sinus fistula, other conditions involving the cavernous sinus and a decompensated phoria.
Material and methods
All patients had a full neuro-ophthalmological evaluation at their initial presentation and at every subsequent visit in our specialist neuro-ophthalmology clinic supervised by a senior consultant neuro-ophthalmologist (JFC) and they also had a full examination by an orthoptist. Personal particulars the exact time of onset of symptoms were noted and any history of vascular risk factors, trauma and myasthenia documented. The nature of diplopia, whether vertical, torsional or horizontal was noted, the angle in prism diopters recorded, and torsional deviations were measured in degrees. These measurements were taken at first presentation to the clinic, at one month, three months and at six months thereafter. On follow-up, the time taken for the resolution of diplopia if this had occurred was noted, and such patients usually discharged, but all unresolved cases were followed up until the end of the six month study period.
Our isolated fourth nerve palsy patients with or without vascular risk factors were not routinely advised to have neuro-imaging at initial presentation unless there was a history of trauma, when imaging was mandatory. Those of our patients who developed other neurological problems during the course of the study were scanned, and all patients with unresolved fourth nerve palsies were also advised to have a scan at the end of the study period.
Results
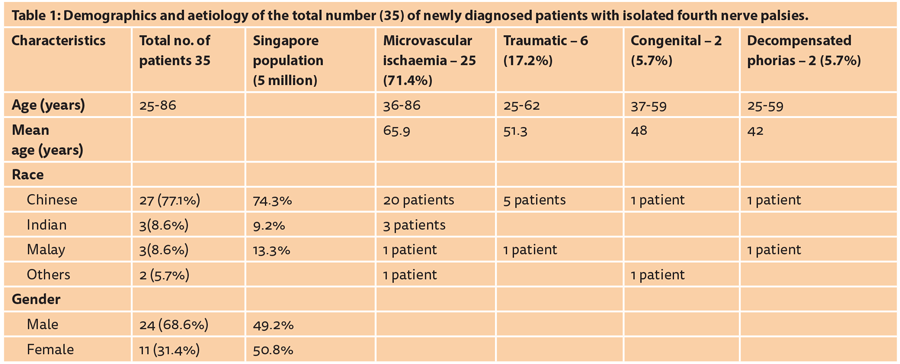
As can be seen in Table 1, the average age of our patients was 65.9 years with a similar mean for the microvascular ischaemic cases. Demographic details and the aetiology in our patients are documented in Table 1. As can be seen in the fourth column 25 (71.4%) of the cases were of presumed microvascular ischaemic cause as per the definition given in the introduction above.
Table 2 details the risk factors and time to resolution in the 25 patients with a presumed microvascular ischaemic aetiology.

It should be noted that hyperlipidemia found in 72% of cases was the commonest risk factor found, followed by hypertension in 64%, and multiple factors were found in 60%. The time for resolution of the palsy or absence thereof in all categories is also shown. In the last column complete resolution occurred in 64% of cases (column 3).
As can also be seen in Table 2 column 5 there were nine (36%) unresolved cases, all of whom had HL as a risk factor, four (16%) having it alone and five (20%) had more than one such factor. Of these nine patients who did not recover, five had multiple risk factors, one had in addition Parkinson’s disease, another trigeminal neuralgia and one was recurrent.
The time to resolution can also be seen in Table 2’s last column, the earliest resolution occurring around three weeks and the latest six months, the average time being 3.4 months.
Neuro-imaging
Brain scans (five CTs and seven MRIs) were performed in 12 of the 25 presumed microvascular ischaemic patients, two of these being younger than average, aged 36 and 45, another had a recurrent palsy, two had atrial fibrillation on Warfarin, another had Parkinson’s disease and one was performed to rule out metastases from breast cancer. Five of the scans were ordered during the patient’s admission to another hospital before referral to our clinic, one of these patients aged 58 with no vascular risk factors had additional MRI and MR angiograms performed which were normal, and complete resolution of this palsy was seen in six months. None of these scans demonstrated any abnormality related to the fourth CN palsy and only three showed brain microvascular ischaemic lesions. The palsy in seven of these 12 scanned patients resolved over a period of one to six months; and the five who did not resolve all had HL as a vascular risk factor, and three had additional risk factors.
Nine of 13 presumed microvascular ischaemic patients who were not scanned had complete resolution over an average period of 3.4 months and for one of these aged 49 having no vascular risk factors the time to complete resolution extended to the full six months. Of the four who did not resolve over the study period, two had multiple vascular risk factors, one had HL alone, one was recurrent; therefore all had HL as one of their risk factors.
Treatment
All patients were treated at the outset if necessary by occlusion, followed by Fresnel prisms pending recovery, and unresolved cases were eventually prescribed ground-in prisms in their spectacles.
Of the six patients with traumatic palsies, three resolved over three, five and eight months respectively, the other three were ordered ground-in prisms pending final review at one year post trauma. The two decompensated phoria patients and the two congenital cases were also prescribed such prisms.
The discussion below only deals with the presumed microvascular ischaemic patients.
Discussion
A recent prospective report on presumed microvascular isolated ocularmotor CN palsies is a multicentre study from 10 centres in the USA conducted over a period of 18 months and published in 2013 [2]. The authors report on 109 patients aged 50 years or older, including two Asian (country of origin not given), 25 of whom had isolated fourth palsies but the aetiology of these fourth palsies is not separately identified. We here report the same number (25) of isolated fourth CN palsies seen over six months in one neuro-ophthalmology unit in Singapore, thus it appears that isolated ischaemic fourth palsies are either much more prevalent in a non- Caucasian population, or it may be that such patients are not being referred to neuro-ophthalmology clinics in the US. Both studies presume microvascular aetiology in accordance with the definition given earlier. The discrepancy may be associated with the higher incidence of vascular diseases in Asian populations, and it has been reported that the pattern of neuro-ophthalmological disease in Southeast Asia is very different to that encountered in the northern hemisphere [3].
An earlier study from Scotland in 1996 [4] reports on 165 ocularmotor palsies seen over nine years, 34 (21%) of which were isolated fourth palsies which are dealt with on their own, nine being traumatic and nine ‘vascular’. Imaging (six CTs and one MRI) was performed in seven patients no abnormality being reported. In the 34 cases, no other cause was discovered and 50% resolved spontaneously over a median period of three months.
“The majority of isolated fourth cranial nerve palsies in adults are of ischaemic origin and resolve spontaneously; therefore they can be safely observed for about six weeks before neuroimaging is required.”
Brain scans were performed in 12 of our 25 ischaemic patients, whereas brain MRIs were performed in all the USA patients, and by excluding patients with third nerve palsies and those with giant cell arteritis (GCA), they found the incidence of other causes for combined fourth and sixth palsies only 4.7%, but as already mentioned they do not give a figure for the fourths separately as we do in this paper, and we did not consider GCA as a possible cause as this condition is rarely seen in southeast Asia [5].
Only three of our scanned patients (and these were ordered from our clinic), showed brain ischaemic changes, and no other pathology was found to account for the nerve palsies in the other nine scanned patients, seven of which resolved spontaneously.
Nine of our 13 patients who were not scanned also resolved over a period of three weeks to six months, the average period being 3.4 months, six of these having multiple risk factors, two HTN and one had no risk factors. Five of the nine unresolved cases had scans performed, with ischaemic brain lesions reported in three. Of the four unresolved patients who were not scanned, two had multiple risk factors and two had HL alone, these four were not scanned because they had improved hence were unlikely to have a compressive cause.
The commonest vascular risk factor in our patients was HL, found in 72%, followed by HTN in 64%, DM in 32% and ischaemic heart disease (IHD) in 20%, whereas the reported prevalence of HL in Singapore population is 17.4% and that of DM is 11.3% [6]. All nine of our unresolved fourth CN palsies had HL as one of their risk factors, four had HL alone and our three recurrent cases also had HL as a risk factor. Therefore, HL contributes to the majority of the ischaemic fourth nerve palsies in this population, and those patients with HL are also more prone to recurrence and are likely to have a non-resolving palsy.
The extrapontine part of fourth cranial nerve obtains its blood supply mainly from branches of the internal carotid artery via its meningohypopyseal and inferolateral trunks which arise within the cavernour sinus, and its more central portion from the posterior communicating and / or posterior cerebral arteries [7]. Thus there are watershed areas along its course explaining its vulnerability to microvascular ischaemia and perfusion loss when these intraneural microvessels become hyalinised as they will do in patients with DM, HTN and HL.
There is no pathological report available on a fresh microvascular ischaemic fourth nerve palsy, thus the exact nature of the process remains conjectural, but in order to explain its good recovery the process must be one of conduction block or demyelination rather than axonal damage [7]. The exact part of the fourth nerve involved in the process is also undetermined, but most likely to occur in that part between the brain stem and its entry into the lateral wall of the cavernous sinus.
In this paper we report that HL is the commonest aetiological factor in our ischaemic fourth CN palsies, of which we encountered a large number of 25 cases over a six month period. Furthermore, neuroimaging in our patients has not been productive in eliciting a cause other than ischaemia (found in three out of 12 scans), and thus we only advise imaging following a period of observation while awaiting spontaneous resolution which can be expected to occur within three months.
With regard to title ‘presumed misrovascular versus other causes’ as used in the US multicentre study paper [2], this can be misleading because ‘microvascular’ is not a cause in itself, but the cause / pathology is in the microvascular blood supply to the cranial nerve in question, so that the more correct terminology should be ‘presumed microvascular ischaemia versus other causes’.
References
1. Lim SA, Wong WL, Fu E, et al. The incidence of neuro-ophthalmic diseases in singapore: a prospective study in public hospitals. Ophthalmic Epidemiology 2009;16:65‑73.
2. Tamhankar MA, Biousse V, Ying G-S, et al. Isolated third, fourth, and sixth cranial nerve palsies from presumed microvascular versus other causes. Ophthalmology 2013;120:2264-9.
3. Cullen JF. Final Observations of a European Neuro-Ophthalmologist in Southeast Asia. Asian J Ophthalmol 2009;11:25-8.
4. Tiffin PAC, MacEwen CJ, Craig EA, Clayton G. Acquired palsy of the oculomotor, trochlear and abducens nerves. Eye 1996;10:377-84.
5. Cullen JF, Chan BMJ, Wong CF, Chew WCI. Giant cell (temporal) arteritis in Singapore: an occult case and the rationale of treatment. Singapore Med J 2010;51:73-7.
6. Ministry of Health Singapore. Health Facts Singapore 2010:
www.moh.gov.sg/content/
moh_web/homes/statistics/
health_facts_Singapore.html
7. Galtrey CM, Schon F, Nitkunan A. Microvascular non-arteritic ocular motor nerve palsies – what we know and how should we treat? Neuro-Ophthalmology 2015;39:1‑11.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME