Microinvasive surgical approaches to primary open-angle glaucoma (POAG) offer minimally traumatic options for effective intraocular pressure (IOP) reduction in appropriately selected glaucoma patients.
Increases in laser trabeculoplasty rates and wider adoption of glaucoma drainage device filtration procedures, together with the introduction of microinvasive glaucoma surgeries (MIGS), illustrate shifts in surgical practice for both moderate and advanced glaucoma. Trabeculectomy rates remain largely flat, while combined glaucoma drainage device surgery and cataract extraction account for a significantly greater share of total glaucoma filtration procedure rates than they did 10 years ago [1].
Novel-acting second generation MIGS devices and procedures currently in development potentially offer safer, better or simpler surgical interventions, without the complications commonly seen with trabeculectomy. Early-generation MIGS approaches are associated with modest or variable IOP reduction compared with trabeculectomy, limiting wider adoption. To achieve improved efficacy results, three outflow targets or placement strategies are being explored: diverting aqueous to the Schlemm canal; shunting aqueous to the suprachoroidal space; and redirecting aqueous to the subconjunctival space.
Schlemm’s canal and conventional outflow
Non-implantable MIGS procedures for adult open-angle glaucoma (OAG) include endoscopic cyclophotocoagulation, trabeculectomy ab interno (Trabectome, Neomedix) and excimer laser trabeculectomy. An ab interno trabeculectomy procedure using the Trabectome device has the advantage of no implant-related complications, such as corneal or iris damage, scarring or fibrosis beyond the postoperative period, and extrusion or dislocation. The device is used to ablate and remove a 60-degree to 120-degree strip of trabecular meshwork to expose the anterior chamber aqueous directly to Schlemm’s canal and the collector channels, opening a continuous pathway from the anterior chamber to Schlemm’s canal. Cauterisation removes tissue to prevent closure and there is no conjunctival scarring. The procedure is performed through a clear-corneal incision and can be combined with phacoemulsification and intraocular lens (IOL) implantation.
A study of primary outcome results of global Trabectome experience for patients receiving Trabectome procedure, involving a total of 4,659 cases, shows on average about a 30% reduction in IOP and 60% reduction in number of glaucoma medications [2]. Results reflect initial surgeon experience involving a study population of patients with various stages of glaucoma progression, 40% having received prior surgery for glaucoma that failed to sufficiently control IOP. Overall, IOP was lowered by 26% at 90 months (from a baseline of 23.1mmHg to 17.2mmHg), and average number of glaucoma drops reduced by 58%, from 2.6 to 1.1, at 90 months. Study author Sameh Mosaed concluded that the Trabectome procedure may be considered for initial therapy and for end-stage patients who have failed to respond adequately to other surgical and medical treatments.
A single-surgeon evaluation of one-year clinical outcomes of Trabectome and trabeculectomy for OAG reported similar IOP lowering effects. In both treatment groups, mean postoperative IOPs significantly decreased from baseline throughout the entire 12 months: mean postoperative IOPs at 12 months were 16.6±1.7mmHg for the Trabectome group (mean preop IOP 22.2±7.4mmHg) and 15.7+5.8mmHg for the trabeculectomy arm (mean preop IOP 25.9±8.9mmHg). Medication use decreased from 3.3±0.9 to 1.4±1.1 in the Trabectome group and from 3.1±0.9 to 1.6±1.2 in the trabeculectomy group at 12 months following surgery [3].
iStent
iStent trabecular micro-bypass intraocular stent (Glaukos Corporation) is designed to optimise aqueous humour outflow by creating a patent bypass between the anterior chamber and Schlemm’s canal, increasing outflow through the inferonasal quadrants and significantly lowering IOP in patients with mild to moderate open-angle glaucoma. The iStent system consists of a surgical-grade nonferromagnetic titanium micro-bypass stent preloaded in a single-use sterile inserter and is heparin coated. The inserter has rotatable grip and reacquisition capability to allow manipulation and micro-targeted placement into Schlemm’s canal. The stent has a single-piece design, measuring 1.0mm in length, 0.33mm in height, with a snorkel (inlet) length of 0.25mm and snorkel bore diameter of 120µm.
The iStent is indicated for use in conjunction with cataract surgery for the reduction of IOP in adult patients with mild to moderate OAG currently treated with ocular hypotensive medication. Pivotal results from a prospective, randomised, multicentre 12-month study, outlined in the package insert, show that 68% of patients who received iStent (n=123) achieved target IOPs ≤21mmHg without use of hypotensive medication compared with 50% of those undergoing cataract surgery alone (n=117) (p=0.004). For IOP reduction ≥20% without glaucoma medications at 12 months, outcomes were 64% for the iStent treatment group and 47% in the cataract surgery alone arm. Implantation of iStent was successful in the majority of cases. Intraoperative complications included iris touch (n=8, 7.1%), endothelium touch (n=1, 0.9%), intraoperative stent removal and replacement (n=1), failure to implant stent (n=1) and stent malposition (n=1). Postoperative adverse events occurred at a low incidence in both groups and were representative for the elderly surgery population evaluated in the study.
Five-year follow-up results from a prospective case series of 19 subjects undergoing cataract surgery with a single iStent show IOP was reduced 16%, from 19.4 to 16.3mmHg, and medication use was lowered to 0.8 from 1.3, with 42% of patients off medications entirely [4].
Registration studies are underway evaluating iStent Inject, a second-generation trabecular bypass implant which can be preloaded with multiple stents and which is pushed straight through the trabecular meshwork into Schlemm’s canal. Two case series evaluating iStent inject (two stents) with or without phacoemulsification show pressure lowering of 36-40% at one year and medication use lowered by one agent [4].
Hydrus Microstent
The Hydrus microstent (aqueous implant, Ivantis) is an investigational intracanalicular scaffold for the treatment of POAG, implanted using an ab interno approach during cataract surgery. The device consists of a flexible Nitinol scaffold with windows along its walls to dilate Schlemm’s canal and allow aqueous humour to flow through. The Hydrus implant is a crescent-shaped device intended to be a permanent implant placed through the trabecular meshwork into Schlemm’s Canal, immediately following placement of a monofocal IOL, in glaucoma patients undergoing cataract surgery. The Hydrus IV clinical trial is a 24-month phase III study evaluating safety and effectiveness of the Hydrus device for lowering IOP in glaucoma patients undergoing cataract surgery, with an estimated primary completion date of January 2016. Hydrus III is a 12-month study comparing Hydrus microstent to iStent trabecular micro-bypass stent for lowering IOP in glaucoma patients having cataract surgery.
Figure 1.

Figure 2.

Figure 3.

Figure 4.

Figure 5.
Figures 1-5: XEN Gel Stent illustrations, images courtesy of Aquesys.

Figure 6: XEN Gel Stent inserter, image courtesy of Aquesys.

Figure 7: iStent inject stent, image courtesy of Glaukos.

Figure 8: Illustration of eye with MicroShunt, image courtesy of InnFocus.

Figure 9: MicroShunt placed on finger for scale illustration, image courtesy of InnFocus.
Suprachoroidal outflow
Targeting suprachoroidal outflow appears a promising MIGS approach for the treatment of glaucoma, offering potential for best-IOP-lowering efficacy based on underlying physiological mechanism, noted Steven D Vold in a US Food and Drug Administration workshop conference. Preliminary safety results from international experience are in line with those seen for other MIGS devices. Supraciliary stenting creates a conduit to the suprachoroidal space, allowing for a ‘controlled cyclodialysis’ that is repeatable and permanent to overcome ciliary body flow resistance. Ab interno supraciliary devices that target the uveoscleral outflow mechanism to lower IOP currently under investigation include the CyPass Micro-Stent (Transcend Medical) and iStent supra stent (Glaukos).
CyPass Micro-Stent
The CyPass Micro-Stent is a supraciliary polyimide tube with a through-lumen designed to create a continuous outflow path from the anterior chamber to the suprachoroidal space by directing aqueous fluid from the front into the back of the eye. The CyPass device is implanted into the supraciliary space through a small corneal incision. It measures 6.35mm in length, has a largest external diameter of 0.51mm, with a 300µm lumen and 76µm fenestrations along its distal end. The stent is loaded on a guide wire of the surgical insertion device. On positioning, the device is secured by retention rings at its proximal end. There are several registered clinical trials underway or completed: COMPASS (US), a study of CyPass Micro-Stent for lowering IOP in glaucoma patients undergoing cataract surgery; CyCLE (EU), a multicentre study designed to evaluate the safety, effectiveness and clinical experience of the CyPass in patients with glaucoma; and DUETTE (EU), a completed study evaluating CyPass Micro-Stent as a stand-alone therapy in patients on glaucoma hypotensive medications.
In the latter study, involving 65 refractory glaucoma patients, IOP was lowered from a mean pretreatment IOP of 24.5mmHg to 16.8mmHg at one year, medication use decreased to 1.5 at one year from a mean pretreatment drop use of 2.2. Nine patients required supplementary glaucoma surgery [5]. Two-year results from CyCLE study evaluating CyPass Micro-Stent for the surgical treatment of OAG when implanted in conjunction with cataract surgery (82 subjects remained in study at 24 months) show minimal complications with substantial lowering of IOP and / or use of IOP-lowering medications. In the cohort with baseline IOP greater than 21mmHg (n=23), mean IOP decreased from 25.5 to 15.8mmHg (change, -37%±19%), and mean medication use was lowered from 2.2 to 1.0±1.1 at two years [6]. The most common adverse events were transient hypotony (15.4%) and micro-stent obstruction (8.8%); 11% of subjects required secondary filtering glaucoma surgery.
iStent supra
iStent supra is a new suprachoroidal stent that is placed ab interno with retention rings for stability. Amongst a number of ongoing studies, one trial is evaluating 12-month outcomes in OAG subjects with one prior trabeculectomy treated concurrently with one suprachoroidal stent and two trabecular micro-bypass stents and a postoperative prostaglandin. Twelve-month outcomes in subjects (n=42) uncontrolled on two hypotensive medications and treated with ab interno implantation of iStent supra (Model G3) and postoperative administration of travoprost show that 98% met the primary efficacy endpoint of ≥20% IOP lowering with a reduction in medication use [7].
SOLX Gold Shunt
SOLX Gold Shunt is a single-use 24-karat gold microimplant being evaluated as a pressure-lowering device for refractory glaucoma (i.e. where medical and conventional surgical treatments have failed). The device is approved for use in Canada as well as in several European countries.
Subconjunctival outflow target
XEN gel stent
The XEN gel stent (Aquesys) is a new, soft and permanent ab interno collagen-derived gelatin implant designed to promote aqueous drainage to the subconjunctival space, with minimum conjunctival tissue disruption and restricted flow to avoid hypotony [8]. It is intended to reduce IOP in patients with POAG where previous medical treatments have failed. The XEN gel stent measures 6mm long, and is injected through a small self-sealing corneal incision using a preloaded injector. Test results show that the implant does not occlude inside the lumen and the implant material does not appear to cause tissue reaction in the eye.
On implantation, XEN gel stent creates a diffuse outflow of aqueous from the anterior chamber into the non-dissected tissue of the subconjunctival space. Potential benefits, according to Aquesys, include: significant and sustained IOP reduction through the subconjunctival outflow pathway; minimally invasive subconjunctival approach bypasses all potential aqueous outflow obstructions; a soft gelatin material minimises complications related to synthetic materials; and significant ocular tissue is available following the procedure, which means that other additional potential treatment options remain available for future consideration if needed. Ab interno blebs also lower the risks associated with ab externo formed blebs such as bleb leaks and infection.
A prospective, non-randomised study evaluating XEN 45 micron implant in combination with a preoperative mitomycin C injection for the treatment of glaucoma (n=98 patients) found a 41% mean IOP reduction at 24 months (from medicated mean baseline of 22.4mmHg to 13.2mmHg, patients were not washed out) [9]. Investigators reported that there were no bleb-related complications, wound leaks, persistent hypotony, vision loss ≥2 lines, or device explants. XEN gel stent is indicated for use with or without phacoemulsification cataract surgery and the implant was termed a MIGS Plus device by Ike Ahmed (Canada), minimally invasive glaucoma surgery with strong pressure-lowering efficacy (Table 1).

InnFocus Mirco-Shunt (formerly MIDI Arrow)
The InnFocus Micro-Shunt glaucoma drainage implant, developed in collaboration with the University of Miami’s Bascom Palmer Eye Institute, is designed to quickly and simply shunt aqueous humour from the anterior chamber to a diffuse bleb without the use of a scleral flap, maintaining low IOP levels in the mid to low teens. The implant is made of ultra-stable and highly durable SIBS material, and consists of an extremely small micro-tube that shunts aqueous to a subconjunctival / sub-Tenon flap. Implantation time, using an ab externo subconjunctival / sub-Tenon’s access, is estimated at 15 minutes.
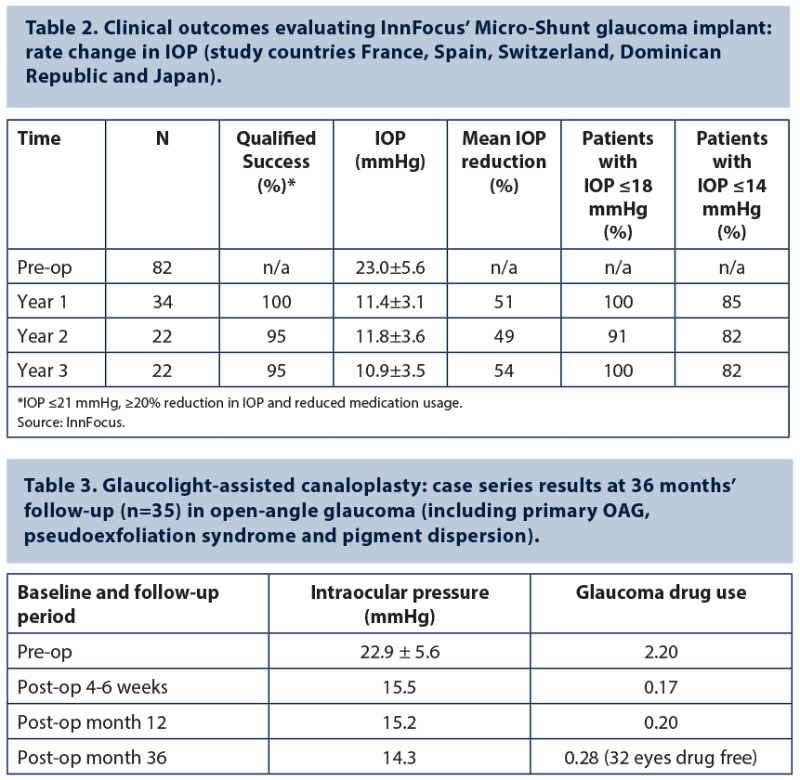
Outcome data from a series of 82 glaucoma patients implanted (alone or in combination with cataract surgery) with the InnFocus Micro-Shunt show a mean postoperative IOP reduction to below 14 mmHg from a mean preoperative baseline of 23mmHg, and over 70% of patients entirely off eye drops after three years (InnFocus data) (Table 2). A multicentre clinical trial is underway in the United States that will compare the safety and efficacy of experimental device InnFocus Micro-shunt and trabeculectomy over two years in subjects with POAG where IOP is inadequately controlled on maximum tolerated glaucoma drops.
Canaloplasty evaluations
Canaloplasty, involving circumferential viscodilation and tensioning of the inner wall of Schlemm’s canal, is a highly effective and safe treatment for OAG, explained Gábor Scharioth, Aurelios Augenzentrum, Germany and University of Szeged, Hungary, speaking during a symposium at the XXXII congress of the ESCRS in London. This non-penetrating glaucoma procedure is characterised by low complication rates, fast recovery of visual acuity, good long-term results and may be adopted in combination with phacoemulsification.
“The XEN gel stent has been termed a ‘MIGS Plus’ device, minimally invasive glaucoma surgery with strong pressure-lowering efficacy.”
Postoperative IOP results achieved with iTrack-assisted viscocanaloplasty (iScience Interventional) are broadly comparable to those observed with Glaucolight-assisted (DORC) canaloplasty, although the latter procedure reduces the risk of Descemet membrane detachment, Professor Scharioth commented. Thirty-six month follow-up evaluation of 35 Glaucolight-assisted canaloplasties in OAG patients, involving no combined surgery or previous glaucoma surgery, showed excellent outcome results, observed Prof Scharioth (Table 3). Mean postoperative IOP at 36 month follow-up was 14.3±2.4mmHg with 0.28 glaucoma medication use, compared with a mean preoperative IOP of 22.9±5.6mmHg and 2.2 glaucoma medications. No severe complications were observed in this single-centre case series.
“Canaloplasty seems to be able to increase outflow facility and reestablish the natural aqueous outflow pathway,” added Prof Scharioth. “Preventing filtering bleb formation reduces complication rates and increases the long-term success of glaucoma surgery. Canaloplasty has potential as a primary treatment option for both moderate and advanced glaucoma.”
Results from a retrospective study evaluating canaloplasty surgery, with and without combined phaco, in 64 eyes from 48 patients show good control overall with a mean follow-up of 33 months: mean IOP was lowered from a preoperative level of 19.4±5.9mmHg to a mean IOP at final follow-up of 16.2±9.3 (p=0.0021); mean number of medications at final follow-up was 0.7±0.3 compared to a preoperative mean of 2.0±1.1 [10]. The commonest complications were hyphema on postoperative day one in 37 eyes (57.8%) which resolved by the one week visit, sub-Descemet membrane haemorrhage in four eyes (6.3%), which resolved over several months, and IOP spike >10mmHg on day one in 10 eyes (15.6%).
Set realistic expectations and appropriate targets
Second-generation MIGS devices potentially offer earlier surgical options to help arrest glaucomatous progression and reduce associated morbidity for patients with mild to moderate glaucoma undergoing cataract surgery, reflecting a step-wise management approach in glaucoma practice. The treatment goal using MIGS procedures is to lower IOP and reduce medication burden in appropriately selected cases where visual field loss is minimal. Available evidence supports successful IOP-lowering outcomes. It is claimed that some newer MIGS placement strategies may come close to providing trabeculectomy-like IOP lowering effects, although no controlled clinical trial has been done comparing the effectiveness of MIGS procedures versus conventional filtering surgery without concurrent phacoemulsification surgery.
Advocates caution that MIGS procedures are not a replacement for trabeculectomy or tube shunts and that they should be avoided in glaucoma patients with advanced visual field loss and uncontrolled IOP. Henry D Jampel (John Hopkins University School of Medicine, Baltimore, USA) disclosed his wish list for future advances in surgical therapy at last year’s Glaucoma Subspecialty Day meeting at the American Academy of Ophthalmology annual meeting: less invasive, more elegant and conjunctiva-sparing surgery that reduces IOP below 15mmHg. Ongoing clinical investigations will provide a better understanding of the relative risk reward profiles and long-term outcomes of different MIGS outflow targets, as well as supporting justification for possible expanded indications.
References
1. Buys YM, Szigiato AA, Trope GE, et al. Trends in glaucoma surgical procedures in Ontario 1992-2012. ARVO 2015 annual meeting abstract #2659, posterboard number AO227.
2. Mosaed S. The first decade of global trabectome outcomes. Clinical & Surgical Ophthalmology 2014;32(1):21-9.
3. Nakano E, et al. One-year clinical outcomes of trabectome and trabeculectomy for open-angle glaucoma. ARVO 2015 annual meeting abstract #2658.
4. Le K, Saheb H. iStent trabecular micro-bypass stent for open-angle glaucoma. Clinical Ophthalmology 2014;8:1937-45.
5. Nguyen Q, Hoeh H, Rau M, et al. Long-term results of supraciliary micro-stent implantation in patients refractory to topical glaucoma therapy. Presentation at the 2014 annual meeting of the American Academy of Ophthalmology, Chicago, IL, USA.
6. Höh H, Grisanti S, Grisanti S, et al. Two-year clinical experience with the CyPass micro-stent: safety and surgical outcomes of a novel supraciliary micro-stent. Klin Monbl Augenheilkd 2014;231(4):377-81.
7. Jünemann A. Twelve-month outcomes following ab interno implantation of suprachoroidal stent and postoperative administration of travoprost to treat open angle glaucoma. Presentation at the XXXI Congress of the ESCRS, 5-9 October 2013, Amsterdam, Holland.
8. Lewis RA. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cataract Refract Surg 2014;40(8):1301-6.
9. Reitsamer H. Early results of a minimally invasive, ab-interno gelatin stent in combination with a preoperative mitomycin C injection for the treatment of glaucoma. Paper presentation at the XXXII Congress of the ESCRS, 13-17 September 2014, London, UK.
10. Chen H, WuDunn D, Cantor LB. Long-term clinical results of canaloplasty in open-angle glaucoma. ARVO 2015; Abstract #2683, posterboard number A0251.
COMMENTS ARE WELCOME