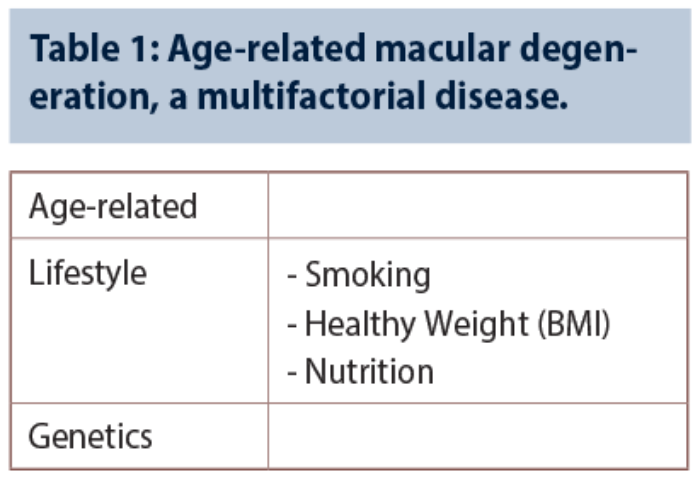
Age-related macular degeneration (AMD) is a multifactorial condition influenced by genetics and lifestyle factors (Table 1). This article outlines several recent advances in AMD genetics, as well as evolving therapeutic concepts and established practical measures for the treatment and / or prevention of advanced macular degeneration.
Genetic risk factors for macular degeneration
Genetic predisposition is estimated to account for 45-70% or more of incident cases of AMD. High odds ratios have been found for chromosome region 1q31, which carries the gene for complement factor H (CFH), and chromosome region 10q26, which includes the ARMS2/HTRA1 genes. Single nucleotide polymorphisms (SNPs) in genes coding for alternative complement pathway proteins are strongly associated with risk for onset of geographic atrophy (GA) and neovascular AMD subtypes; there is for instance a strong association of AMD with variants in the complement component 2 (C3) gene.
Johanna Seddon and colleagues at New England Eye Center have developed and validated a risk prediction calculator for progression of macular degeneration (www.seddonamdriskscore.org.)
It is based on 10 common and rare genetic variants related to AMD and incorporates demographic, behavioural and macular characteristics. The selected genetic variants include K155Q in the C3 gene, three common and rare variants in CFH , and six common variants in ARMS2/HTRA1, CFB, C3, C2, COL8A1 and RAD51B. Risk prediction evaluations, involving survival analysis of subjects participating in the Age-Related Eye Disease Study, revealed that 80% of incident AMD is attributable to genetic factors after adjusting for baseline macular phenotypes [1]. Use of a 10 genetic loci model could differentiate between low, medium and high risk of progression to advanced AMD. Investigators say that the risk calculator may be useful for initial screening to identify high-risk individuals, collaborative disease management and clinical research.
It was shown in 2013 that there is an association between rare genetic variants in the complement factor I gene (CFI) and advanced AMD [2]. The Factor I (FI) protein is considered an important mediator of complement regulation by cleaving C3b and C4b in the presence of its cofactors [3]. A study evaluating serum FI as a potential biomarker for advanced AMD found that low serum FI levels are strongly associated with rare CFI variants and with advanced AMD [4]. Identifying individuals with CFI mutations associated with low FI levels might allow more precise targeting of patient populations most likely to benefit from complement inhibitor therapy, investigators observed.
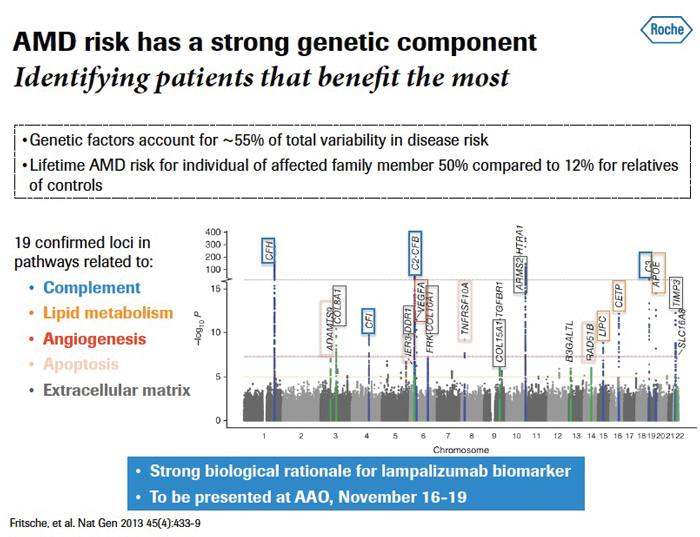
Figure 1: AMD and genetics.
Does genetic predisposition influence treatment response? Results from a study presented at ARVO 2015 show the genetic effects of 19 known AMD risk loci are not associated with either rapid progression or good treatment response in a small sample dataset [5]. This suggests that unknown or rare genetic factors may be at play in influencing variability in progression and vision response to antiangiogenic treatment. Good treatment response was defined as a three line vision gain from baseline on the Snellen vision chart, prolonged or persistent (at least six months) absence of subretinal fluid (SRF) and absence of intraretinal fluid (IRF) on optical coherence tomography (OCT), and presence of unchanged small intraretinal cysts with no recurrence of SRF. Poor response was defined as a loss of three lines of vision, persistent SRF and IRF, and disciform scar formation.
A retrospective chart review involving a small series of 72 AMD patients found that those with fewer high-risk alleles in the CFH gene had better visual and anatomic outcomes after antivascular endothelial growth factor (anti-VEGF) treatment. Better visual acuity improvements were noted for low-risk CFH genotypes, and a greater reduction in central foveal thickness was seen in patients having a low CFH risk score [6].
Patients with GA and intermediate AMD had the highest levels of complement activation, according to findings from a retrospective case-control study in Europe evaluating levels of complement activation amongst a diverse patient population (887 patients and 1004 controls) with early, intermediate or advanced AMD [7]. Moreover, patients treated with anti-VEGF therapy had lower complement activation levels.
Therapeutic targets for dry AMD
Inflammation and angiogenesis are two principal mechanisms involved in the pathogenesis of AMD.
Initial clinical signs of dry AMD are anatomic, such as drusen and pigmentary changes, with symptoms of visual function impairment. The pathology of geographic atrophy is characterised by defined areas of retinal pigment epithelium (RPE) atrophy, the fovea is generally unaffected until late stages, and the progression rate is variable across patient groups. Age, metabolic and environmental factors such as smoking, increased plasma high-density lipoprotein cholesterol and body mass index (BMI), as well as bilateral AMD, can contribute to an increase in progression rates. In geographic atrophy, environmental exposure and genetic risk can trigger complement mediated inflammation that may lead to retinal cell death [8]. Hyperactivation of the alternative complement pathway is associated with complement dysfunction in GA.
Complement inhibition, suppression of inflammation, visual cycle modulation/reduction of toxic by-products, neuroprotection and stem cell therapy are all therapeutic concepts being explored for dry AMD, to reduce progression or potentially restore some useful vision. Clinical trials in dry AMD use fundus autofluorescence (FAF) to assess progression, as dry AMD involves slowly progressive changes and there may not be significant visual acuity change during the evaluation period of most clinical trials.
Lampalizumab for GA associated with AMD
Lampalizumab (anti-Factor D Fab, Roche) is a humanised monoclonal antibody fragment targeting complement factor D. It is designed to inhibit complement activation and chronic inflammation in tissues. Complement factor D is a member of the trypsin family of peptidases and is a component of the alternative complement pathway.
Administered by intravitreal injection, lampalizumab treatment was shown in the MAHALO phase II clinical trial to have a positive effect in reducing progression of geographic atrophy in patients with advanced dry AMD. The primary endpoint in MAHALO was change of GA area from baseline to month 18 compared with control, as assessed with fundus autofluorescence. Treatment with lampalizumab administered monthly was associated with a 20.4% reduction rate in the area of GA at 18 months (p-value less than pre-specified significance level of 0.2), with high efficacy observed in a subpopulation with an exploratory biomarker CFI. In CFI positive subjects treated with monthly lampalizumab, investigators reported a reduction of 44% in GA area progression at month 18 vs. sham control, while there was an 18% reduction in GA area progression in the lampalizumab every-other-month CFI-positive subpopulation vs. sham. There was no apparent treatment effect in subjects who were negative for the CFI biomarker.
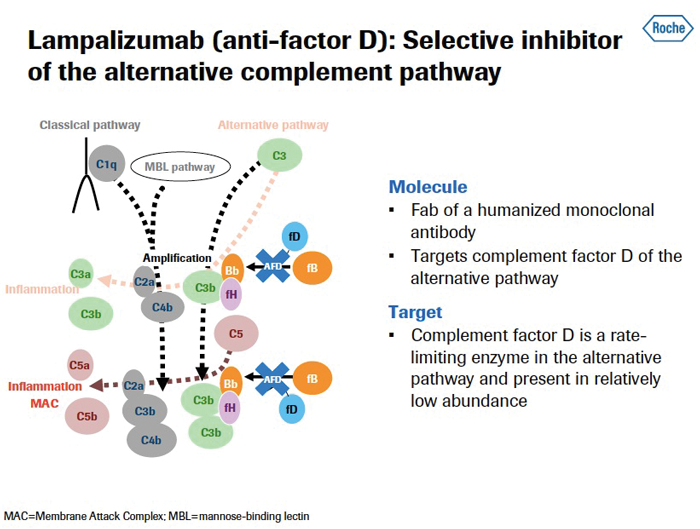
Figure 2: Lampalizumab mechanism of action / alternative complement pathway.
Data presentations from early MAHALO investigations show that patients who were carriers for the risk allele for CFI had faster progression of GA area compared with non-carriers of the CFI risk allele (49% greater increase from baseline in GA area growth over 18 months, change from baseline 4.169mm² vs. 2.792mm² in non-carriers) [9].
The best test of the validity of results from subgroup analyses is of course replication of findings in confirmatory studies. Some recent studies have failed to reproduce an association between growth of geographic atrophy and the complement factor I locus [10,11], with additional larger and longer follow-up studies needed to confirm or clarify the association of CFI with GA progression.
Two identically-designed phase III pivotal randomised clinical trials are currently enrolling globally, named Chroma and Spectri, that are designed to evaluate 10mg intravitreal lampalizumab administered every four weeks and every six weeks vs. sham control in both CFI positive and CFI negative patient populations (936 participants in each trial). Inclusion criteria are bilateral GA without choroidal neovascularisation (CNV), best corrected visual acuity (BCVA) 20/100 or better, and total lesion size between ~1-7 disc areas. The primary endpoint is mean change in GA area from baseline to week 48, as assessed by retinal imaging. Study participants completing the trial will be eligible to enroll in an open-label extension study through an overall study duration of two years. Roche has a provisional expected filing date of 2017+ for registration submission, subject to readout of efficacy and safety data from these pivotal clinical trials.
Natural history data on visual function decline in GA patients remains relatively limited. Sponsored by Roche, Proxima is a longitudinal, non-interventional, observational study designed to contribute further to the understanding of visual function in a broad population of GA patients, and also to gather additional scientific data on the relationships between genetics and GA progression. Patients will be followed every six months for up to five years, involving assessment of visual function, GA lesion growth and genotyping. Research into the natural history of early and intermediate AMD, including 20-year progression rates, shows that AMD pathology tends to relentlessly progress over the long term [12]. Eyes with no or early AMD changes show low rates of progression to late AMD.
Tissue repair or regeneration using stem cell therapy
Seminal studies evaluating human embryonic stem cell (hESC)-derived RPE transplantation in patients with AMD (n=9) and Stargardt’s macular dystrophy (n=9) found 72%, or 13 of 18 patients, showed increasing subretinal pigmentation consistent with transplanted RPE at a median of 22 months’ follow-up [13]. Best corrected visual acuity improved in 10 eyes, with no similar improvements seen in untreated fellow eyes.
Steven D Schwartz (Jules Stein Eye Institute, Los Angeles, USA), speaking during the RCOphth 2015 Retina Day meeting in Liverpool in May, noted that this is the first study to demonstrate the mid- to long-term safety, survival and possible biologic activity of pluripotent stem cell progeny into humans with any disease. Results suggest that hESC-derived cells could provide a potential new source of cells for the treatment of a variety of unmet medical conditions caused by tissue loss or dysfunction.
Reducing the risk of AMD
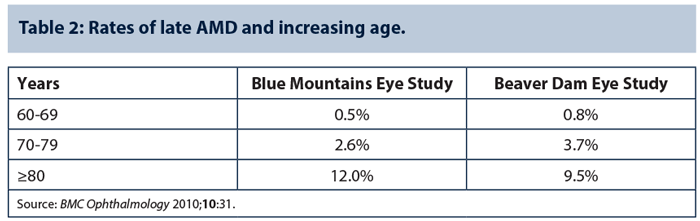
Speaking in a spotlight symposium on wet and dry AMD at the annual meeting of the AAO in Chicago 2014, Emily Y Chew (National Eye Institute / National Institutes of Health, Bethesda, USA) discussed the role of nutritional supplements and lifestyle strategies to reduce the risk of AMD. Advancing age (Table 2), current smoking, previous cataract surgery and a family history of AMD are recognised as consistent risk factors for AMD [14], and there are strong associations between AMD and cardiovascular risk factors and higher BMI. An examination of cross-sectional and prospective studies shows less than a two-fold increase in AMD in those with the highest BMI compared with those having the lowest BMI.
Vitamin supplementation recommended for those with intermediate or late AMD
It was reported over two decades ago that a diet rich in fruit and vegetables with vitamins A and C was inversely associated with AMD prevalence [15]. AREDS supplementation with lutein / zeaxanthin is effective in reducing progression to advanced AMD compared with AREDS supplements with beta-carotene. Patients who would benefit the most from lutein / zeaxanthin supplementation are those with the lowest dietary intake levels of lutein / zeaxanthin. AREDS supplementation with lutein / zeaxanthin is effective in reducing progression to neovascular AMD and not to central GA compared with AREDS supplements with beta-carotone. Lutein and zeaxanthin supplementation may also possibly increase retinal macular pigment optical density response in certain genetic variants, with study findings showing association between genetic variants and response to carotenoids supplementation [16].
Should ophthalmologists recommend genetic testing for AMD and, more specifically, should ophthalmologists conduct genetic testing for AMD prior to prescribing AREDS supplements? It was noted that AREDS supplements are found to be beneficial in all genotype groups, with no evidence of harmful effects. Moreover, there is no other treatment available other than AREDS supplements for intermediate AMD and nothing for early AMD. People should be advised to maintain a healthy lifestyle, to exercise, avoid cigarette smoking and eat a good varied diet. Persons with intermediate AMD or late AMD in one eye should consider taking AREDS/AREDS2 supplements. Avoid genetic testing for AMD except for research purposes, argued Dr Chew.
The role of genetic profiling in AMD is a topical debate. Some practitioners believe that testing for AMD-related genes may influence treatment practice for dry AMD in the future, assuming proven preventative treatments emerge.
“Age-related macular degeneration (AMD) is best thought of as a group of genetically- and environmentally-determined retinal degenerative conditions,” commented Professor John Marshall, UCL Institute of Ophthalmology, London, speaking in a telephone interview with the author. “Screening is helpful for clinical research purposes but at the moment premature for therapeutic intervention and probably would not be considered cost-effective in the UK. Addressing known risk factors for advanced forms of AMD is nonetheless hugely important to help prevent or ameliorate vision impairment in later life.”
Mediterranean-style diet and regular physical activity may be protective
Recently presented research findings on genetic risk factors in AMD, nutrition and inflammation support established evidence on the protective or preventive effects of a nutritious diet and of regular physical activity with regard to AMD onset and progression risks.
A Mediterranean-type diet (e.g. dark green leafy vegetables, fruit and olive oil) may be protective for neovascular AMD and large drusen [17]. A population-based study of age-related eye diseases among 963 elderly residents of Bordeaux in France found daily consumption of olive oil was significantly associated with a decreased risk of late AMD (odds ratio of 0.32, 95% confidence internal: 0.15-0.67) [18].
More needs to be done to encourage people to improve their diet by following national dietary guidelines on regular intake of fresh fruit, vegetables and fish, especially for those at risk of AMD [19,20]. A study into use of eye vitamins for macular degeneration in the US population seven years after publication of the landmark AREDS study found utilisation by those at risk for end-stage AMD is inadequate. Just 4.7% of adults aged 40 years or more in this representative sample were using eye health vitamins, while only 16.6% of those at risk of end-stage AMD were taking vitamin supplements.
Evidence from multiple separate national studies demonstrates that regular physical activity may have a protective effect against AMD, highlighting the public health message that it’s best to stay active always.
Discussion
An evaluation of the long-term (15-year) cumulative incidence and progression of intermediate drusen, involving the Blue Mountains Eye Study cohort, confirmed that increasing age and genetic susceptibility, confirmed by the presence of CFH and ARMS2 risk alleles, were associated with greater risk of developing intermediate drusen [21]. The greatest risk of progression to late AMD was found in those with intermediate drusen and retinal pigment abnormalities.
Defective immune modulation is a consistent theme in AMD pathogenesis [22]. There is increasing recognition that AMD shares certain common molecular disease pathways with Alzheimer’s disease and other neurodegenerative disorders. Macular degeneration may contribute to cognitive disorders [23,24], while modulation of ß-amyloid aggregation may potentially confer a neuroprotective effect in Alzheimer’s disease, dry AMD and glaucoma [25]. While there are no proven treatments currently for GA secondary to AMD, future therapies offered for dry AMD could well be influenced by specific genetic profiles.
References
1. Seddon JM, Silver RE, Kwong M, et al. Risk prediction for progression of macular degeneration including 10 common and rare genetic variants. ARVO 2015. Abstract #796-D0258.
2. Seddon JM, Yu Y, Miller EC, et al. Rare variants in CFI, C3 and C9 are associated with high risk of advanced age-related macular degeneration. Nat Genet 2013;45(11):1366-70.
3. Wagner E, Kavanagh D, Yu Y, et al. Rare genetic variants in the CFI gene are associated with advanced age-related macular degeneration and commonly result in reduced serum Factor I level. ARVO 2015. Abstract #782-D0244.
4. Kavanagh D, Yu Y, Schramm EC, et al. Rare genetic variants in the CFI gene are associated with advanced age-related macular degeneration and commonly result in reduced serum factor I levels. Hum Mol Genet 2015;24(13):3861-70.
5. Courtenay MD, Cade W, Wang G, et al. Known age-related macular degeneration risk variants are not associated with rapid disease progression or good treatment response. ARVO 2015. Abstract #797-D0259.
6. Shah A, Williams S, Baumal C, et al. Predictors of response to intravitreal anti-vascular endothelial growth factor treatment of age-related macular degeneration. ARVO 2015. Abstract #3788-C0030.
7. Lechanteur Y, Schick T, Groenewoud J, et al. Levels of complement activation differ between stages of age-related macular degeneration. ARVO 2015. Abstract #787-D0249.
8. Yang P, Baciu P, Kerrigan BC, et al. Retinal pigment epithelial cell death by the alternative complement cascade: role of membrane regulatory proteins, calcium, PKC, and oxidative stress. Invest Ophthalmol Vis Sci 2014;55(5):3012-21.
9. Yaspan B, Li Z, Dressen A, et al. A common SNP at the CFI locus is associated with rapid progression of geographic atrophy. ARVO 2014. Poster #2234-A0025.
10. Wurzelmann JI, Lopez FJ, Fries M, et al. SNPs associated with complement factor I do not predict 4-month lesion growth rate in geographic atrophy. ARVO 2015. Poster #2850-C0078.
11. Yehoshua Z, Garcia Filho A, Nunes RP, et al. Association between growth of geographic atrophy (GA) and the complement factor I (CFI) locus. ARVO 2015. Poster #2845-C0073.
12 Hasan J, Agron E, Clemons TE, et al. Twenty-year follow-up of age related macular degeneration in the Age-Related Eye Disease Study. ARVO 2015. Abstract #3792-C0034.
13. Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 2015;385(9967):509-16.
14. Chakravarthy U, Wong TY, Fletcher A, et al. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol 2010;10:31.
15. Goldberg J, Flowerdew G, Smith E, et al. Factors associated with age-related macular degeneration. An analysis of data from the first National Health and Nutrition Examination Survey. Am J Epidemiol 1988;128(4):700-10.
16. Yonova-Doing E, Hysi PG, Venturini C, et al. Candidate gene study of macular response to supplemental lutein and zeaxanthin. Exp Eye Res 2013;115:172-7.
17. Hogg RE, Woodside J, Chakravarthy U, et al. Association of a Mediterranean type diet with age-related macular degeneration in the EUREYE study. ARVO 2015. Abstract #3760-C0002.
18. Cougnard-Gregoire A, Merle BM, Korobelnik JF, et al. Consumption of foods rich in polyphenols and age-related macular degeneration: the Alienor study. ARVO 2015. Abstract #3773-C0015.
19. Backus S, Buitendijk GH, Kiefte-de Jong JC, et al. National dietary guidelines: protection for age-related macular degeneration for the happy few. ARVO 2015. Absract #3762-C0004.
20. Swanson MW. National estimates of eye vitamin use among those at risk of end stage macular degeneration. ARVO 2015. Abstract #3785-C0027.
21. Wang JJ, Joachim N, Kifley A, Mitchell P. Long-term incidence and progression of intermediate drusen. ARVO 2015. Abstract #3793-C0035.
22. Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron 2012;75(1):26-39.
23. Hasan J, Agron E, Clemons TE, et al. Twenty-year follow-up of age related macular degeneration in the Age-Related Eye Disease Study. ARVO 2015. Abstract #3792-C0034.
24. Mandas A, Mereu RM, Catte O, et al. Cognitive impairment and age-related vision disorders: their possible relationship and the evaluation of the use of aspirin and statins in a 65 years-and-over Sardinian population. Front Aging Neurosci 2014;6:309.
25. Parsons CG, Ruitenberg M, Freitag CE, et al. MRZ-99030 - A novel modulator of Aβ aggregation: I - Mechanism of action (MoA) underlying the potential neuroprotective treatment of Alzheimer’s disease, glaucoma and age-related macular degeneration (AMD). Neuropharmacology 2015;92:158-69.
TAKE HOME MESSAGE
-
Genetic predisposition accounts for an estimated 45-70% or more of incident cases of AMD.
-
Complement inhibition, suppression of inflammation, visual cycle modulation / reduction of toxic by-products, neuroprotection and regenerative stem cell therapy are all therapeutic concepts being explored for dry AMD.
-
Vitamin supplementation, a Mediterranean-style diet, and regular physical activity may protect against AMD or lower progression risk for neovascular macular degeneration.
-
Future therapies offered for dry AMD could well be influenced by specific genetic profiles.
COMMENTS ARE WELCOME