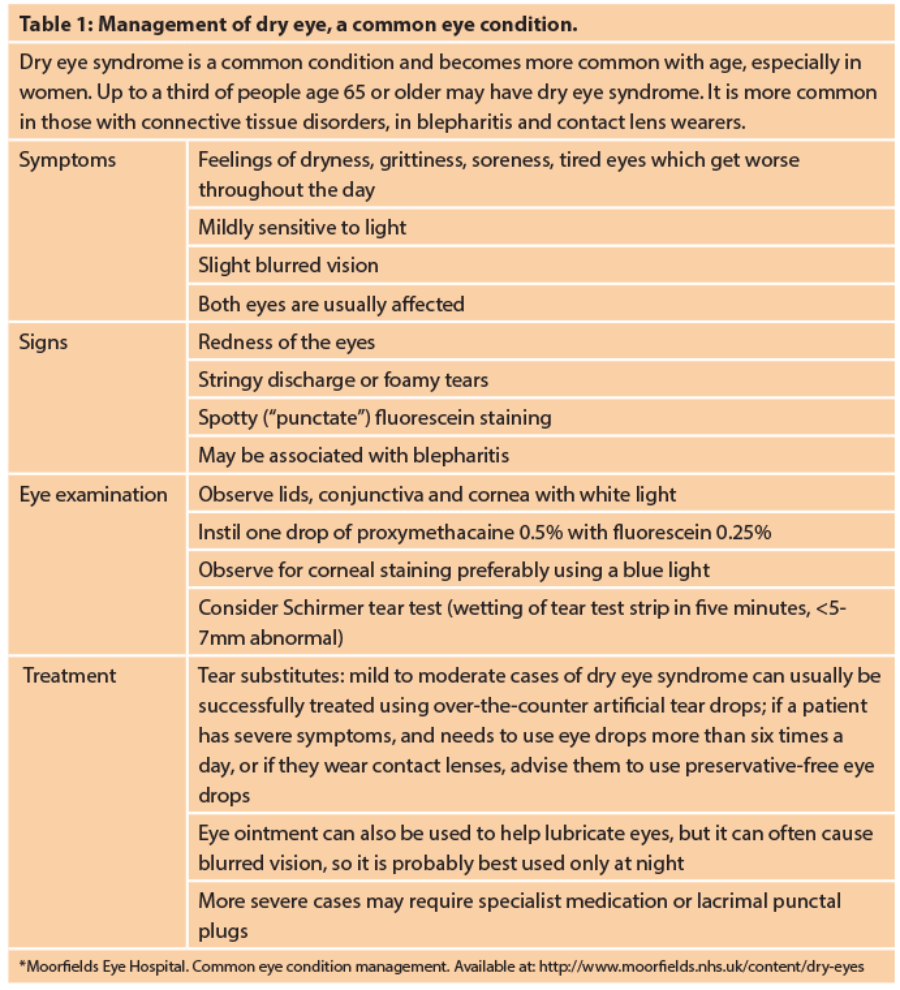
An estimated 344 million people worldwide suffer from dry eye [1]. This chronic syndrome is characterised by a vicious cycle of tear film hyperosmolarity, tear instability and corneal stress, leading to increased friction, inflammation, ocular surface damage and decreased visual acuity. The author reviews emerging developments focused on improving treatment success.
The Tear Film & Ocular Surface Society (TFOS) Dry Eye Workshop II (DEWS II) global consensus report represents the first evidence-based reexamination of multiple aspects of dry eye disease (DED) since the initial international seminal DEWS report issued in 2007. TFOS DEWS II sought over the past two years to update the definition, classification and diagnosis of DED, critically evaluate the epidemiology, pathophysiology, mechanism, and impact of this disorder, address its management and therapy and develop recommendations for the design of clinical trials to assess pharmaceutical interventions for DED treatment.
DEWS II has redefined dry eye as a multifactorial disease of the ocular surface characterised by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play aetiological roles.
Incidence and persistence rates
The prevalence of DED is higher in women and in older people, with a reported 15-33% of people aged 65 years or over impacted by the condition. Approximately 20% of people with DED have severe disease.
A longitudinal study assessed rates of incidence and also resolution / persistence of DED in 3136 subjects (mean age 59 years, 91% female) from the population-representative TwinsUK cohort [2]. Of those without DED at baseline (2310 subjects), two years later 9.8% had incident symptomatic DED, 3.5% had an incident clinical diagnosis and treatment of DED, and 4.1% had incident DED as defined by the Women’s Health Study.
Risk factors associated with incidence of symptomatic DED were female gender, asthma, osteoarthritis, chronic pain syndromes, depression, higher copper intake, lower coffee intake, and increased body mass index. Tear osmolarity at baseline was also associated with incidence of symptomatic DED. Of the 615 subjects with symptomatic DED at baseline, 37.1% showed resolution of symptoms two years later. Factors associated with persistence of symptomatic DED were osteoarthritis, increasing age and female gender. The results suggest that symptomatic DED can be highly variable over time.
Practice patterns
Ophthalmologists continue to prefer the use of traditional dry eye tests in practice, with the most common test being corneal fluorescein staining, followed by tear break-up time and anaesthetised Schirmer’s test, according to a survey of dry eye practice patterns among US ophthalmologists [3]. Sjogren’s disease represents a significant but often overlooked subset of DED patients. Continuing under-referral of dry eye patients for Sjogren’s syndrome work-ups may contribute to under diagnosis of this autoimmune disorder, concluded researchers [3].
Similarities and divergences in self-reported dry eye clinical practice between Australian and UK optometrists were noted by Downie et al. [4]. Optometrists in both regions generally tailored DED therapy to severity, with mild DED predominantly managed with eyelid hygiene and tear supplementation. However, Australian optometrists prescribed topical corticosteroid therapy significantly more often than UK practitioners for moderate (14% vs. 6%) and severe (52% vs. 8%) disease (p<0.05).
Review of recent dry eye literature
New diagnostic techniques and treatment options provide an opportunity for better patient management. For example, TearLab Corporation announced in March 2017 CE Marking for its next-generation in-vitro diagnostics testing platform, offering eye care professionals the ability to assess multiple biomarkers in human tears with a single nanolitre volume tear collection. In a presentation at ARVO 2017, researchers outlined good comparative discrimination results evaluating the fractal dimension (FD) based method to differentiate between normal and dry eye subjects using high speed videokeratoscopy [5].
Milner and colleagues, on behalf of the Cornea, External Disease and Refractive Society (CEDARS) Dysfunctional Tear Syndrome (DTS) Panel, recently presented a consensus compilation of strategies for diagnosis and treatment of DTS [6]. A directed treatment plan is recommended based on a four-stage differential diagnosis and relative severity of the condition to improve patient outcomes.
A differential diagnosis is achieved through patient examination involving a series of clinical assessments and diagnostic techniques. Patients with DTS are classified into one or more of four DTS subtypes, allowing for a directed treatment approach: aqueous deficiency, blepharitis / meibomian gland dysfunction (MGD), goblet cell deficiency / mucin deficiency, and exposure-related DTS. Meibomian gland dysfunction is a leading cause of evaporative DED and is also often present in conjunction with primary aqueous-deficient DED. The authors note that adjustments in lid hygiene, warm compresses and massage may also be necessary for patients with blepharitis / MGD.
Artificial tears, gels, ointments and inserts replenish the tear film, and tears may be conserved via punctal plugs, cautery and moisture chamber eyewear. Anti-inflammatory and immunomodulatory treatment options include ciclosporine, lifitegrast (Xiidra®, Shire Ophthalmics), steroids and nutritional supplements. In clinical practice in the UK, people with severe DED use several drops of artificial tears per day. If the disease does not respond to artificial tears, treatment with other anti-inflammatory formulations and corticosteroids is considered. Punctal plugs remain an option for people with severe DED that does not respond to artificial tears and are often considered after treatment with ciclosporin.
A Cochrane database meta-analysis evaluating the effectiveness and toxicity of over-the-counter (OTC) artificial tear drops for dry eye syndrome concluded that they may be a safe and effective means for treating dry eye syndrome [7]. The literature indicates that most OTC artificial tears produce similar symptomatic relief. The authors noted that additional work is needed to systematically determine if one OTC artificial tear formulation is superior to another, with additional research required to determine if there are advantages to using newer tear lipid-containing OTC artificial tear formulations.
Punctal occlusion aims to block the tear drainage system in order to aid in the preservation of natural tears on the ocular surface. An updated Cochrane systematic review of evidence evaluating punctal occlusion for dry eye syndrome concluded that it was unclear whether punctal plugs are effective for treating dry eye syndrome, i.e. the evidence suggests that punctal plugs do not conclusively improve dry eye symptoms [8]. The review included 18 trials with 711 participants (1249 eyes), most of whom were women, conducted from March 1998 to May 2014, with the search for trials covering publication up to 8 December 2016. The results show that no type of punctal plug used in the trials that were assessed was significantly better than another for relieving symptoms of dry eye. Reviewers noted that it is still unclear if punctal plugs are better than oral treatment (oral pilocarpine) or eye drops such as ciclosporine or artificial tears. The use of punctal plugs is commonly associated with epiphora and, less commonly, with inflammatory conditions such as dacryocystitis.
Clinical evaluations
Results of a phase III, randomised multicentre study demonstrate that the bioprotective trehalose (3%) solution (hyaluronic acid [HA]-trehalose; Thealoz / Duo® / Théalose®, Laboratoires Théa) is at least as effective as HA in the treatment of dry eye syndrome, and is better tolerated than HA. Symptomatic relief with preservative-free HA-trehalose was evident as early as day 35, encouraging longer term treatment compliance [9]. A randomised controlled trial in moderate to severe dry eye showed that an eye drop combining osmoprotectants, carboxymethylcellulose and HA is noninferior to HA. More patients reported less severe stinging / burning, sandiness / grittiness, and painful / sore eyes at month 3 with the multi-ingredient eye drop (p≤0.039) [10].
The SICCANOVE study found that ciclosporine A cationic emulsion (CsA CE, Ikervis®, Santen) was well tolerated and effectively improved signs and symptoms in patients with moderate to severe DED over six months, especially in patients with severe disease who are at risk of irreversible corneal damage [11]. Ciclosporin A cationic solution is a sterile, positively charged, oil-in-water, unpreserved ophthalmic emulsion for the treatment of severe keratitis in adult patients with DED that has not improved despite treatment with tear substitutes. The definition of ‘severe keratitis’ is a corneal function score (modified Oxford scale (CFS)) of 4, a Schirmer score (without anaesthesia) of 2-10mm and an Ocular Surface Disease Index (patient reported outcome (OSDI)) score of 23 or more.
Lifitegrast ophthalmic solution 5% is a new topical medication approved by the United States (US) Food and Drug Administration for the treatment of the signs and symptoms of DED in adult patients. Lifitegrast is an integrin antagonist that decreases inflammation on the ocular surface, thereby improving DED. Results from prospective, placebo-controlled studies in more than 2000 patients demonstrate that lifitegrast was effective for improvement in both the signs and symptoms of DED [12]. A subsequent study evaluating the safety profile of lifitegrast showed that the majority of adverse events were mild and resolved over time.
EvoTears® (Novaliq / Ursapharm) is a multi-dose, preservative-free eye lubricant for stabilisation of the lipid layer commercially available in Europe since 2015. The product offers a new mode of action by acting as a lubricating lipid layer substitute and evaporative barrier for improved tear film stability / quality. A follow-on product development with the addition of omega-3 fatty acids is anticipated. Novaliq’s EyeSol drug delivery technology is based on novel and exclusive water-free excipients which have been established and validated as key ingredients for innovative ophthalmic products. In January 2017 Heidelberg-based Novaliq announced positive topline results of a phase 2 clinical trial evaluating a clear, preservative-free cyclosporine A solution (CyclASol) in adults with moderate to severe DED. Groups treated with CyclASol (0.05% and 0.1%) showed a significant improvement in corneal staining compared with vehicle control over the four-month treatment period (n=207).
A prospective, investigator-masked study assessed the ocular efficacy, tolerance and safety of Cationorm (Santen), a preservative-free cationic ophthalmic emulsion, in comparison with reference treatment 0.18% hyaluronate sodium (HS) solution in patients with bilateral moderate to severe DED with keratitis or keratoconjunctivitis [13]. Cationorm was similar and non-inferior to HS with regards to safety and efficacy for objective signs, but was superior to HS in improving symptoms in patients with moderate to severe DED, including greater alleviation of itching and eye dryness.
Research: novel therapies for dry eye under investigation
Zhang and colleagues emphasised that successful clinical management of dry eye should aim to restore the homeostasis of the ocular surface environment [14]. Few new treatments for DED have emerged over the past decade, supporting the growing need for validated biomarkers and endpoints for dry eye clinical research [15].
Research findings suggest that multiple factors contribute to dry eye and developments remain focused on longer-lasting therapies requiring less frequent dosing. Current dry eye treatments such as steroids, antibiotics and artificial tears provide short-term symptomatic relief, require frequent reapplication and also do not address the underlying causes of dry eye. The National Eye Institute (NEI) in the US recently pointed to several novel therapies under investigation for dry eye.
A phase I/II clinical trial sponsored by TearSolutions Inc. is underway to investigate the use of a topical synthetic lacritin peptide in people with dry eye associated with Sjogren’s syndrome. By restoring a more normal ocular surface condition, lacritin appears to remove triggers that give rise to inflammation. Preclinical study results showed lacritin was effective in promoting tear secretion, restoring ocular surface integrity, and reducing focal infiltration of inflammatory CD4+ T cells in lacrimal glands. Clinical study results are expected in early 2018.
In addition, researchers at Weill Cornell Medical College in New York are exploring factors that influence the ability of corneal nerves to sense basal tear evaporation and other NEI-funded researchers are evaluating approaches that promote the regrowth of corneal nerves. Researchers at Stanford University have developed an implantable device that electrically stimulates the lacrimal gland to produce tears. In addition, it stimulates the nerves linking the brain and sensory neurons in the eye, further prompting the lacrimal gland to produce tears containing lipid, mucins as well as water that more closely resemble the consistency of naturally-occurring basal tears.
The NEI is funding studies to define the molecular composition of the tear lipid layer and to evaluate stem cell therapy to repair the lacrimal glands.
Several OTC dietary supplements containing omega-3s are already available; however, efficacy claims are based on limited, small studies. An upcoming clinical trial is evaluating the benefits of oral omega-3 fatty acids for dry eye, representing the first large, independent, multisite investigation of omega-3s for dry eye.
Danish-based MC2 Therapeutics’ MC2-03 PAD™ Eye Drop (PADciclo™ 0.06% ciclosporin) is currently being investigated in a large phase II trial for treatment of moderate-severe dry eye. The drug candidate is a once-daily formulation of ciclosporin designed for improved penetration and tolerability.
Real-life performance of the unlicensed PADciclo™ 0.06% ciclosporin was evaluated in a retrospective study of dry eye patients (n=22) at Moorfields Eye Hospital, London, with the purpose of verifying key elements in a prospective clinical trial design [16]. Treatment duration ranged from 4 to 52 weeks for the study cohort, while 10/22 patients had used PADciclo for more than 26 weeks. The analysis indicates that PADciclo is effective in reducing corneal staining and global symptoms of dry eye. The global symptom score improved in 13/22 patients with a mean change of 0.7 (p<0.01) and the Oxford scale score reduced in 16/22 patients with a mean change of 0.8 (p<0.001). The response to treatment increased with severity at baseline for global symptoms and corneal staining. Only one patient in this cohort discontinued treatment because of poor tolerance.
Lubricin is an endogenous glycoprotein expressed in areas of high shear stress and friction including the tear film where it binds to and protects tissues of the ocular surface. Lubricin protein deficiency is observed in dry eye patients. ECF843 is a recombinant form of human lubricin protein and potential first-in-class therapeutic in dry eye, recently in-licensed by Novartis from Lubris LLC for ophthalmic indications worldwide (outside Europe). Results of a small phase II study showed ECF843 has the potential to provide instant relief of symptoms and improve signs [17]. In this study, lubricin supplementation achieved greater than a 72% reduction from baseline in foreign body sensation (p<.013), burning / stinging, pain, sticky feeling (p<.0432), blurred vision (p<.0013), and photophobia (p<.011) in at least one eye, together with an improvement in signs of dry eye within 28 days.
Instant relief of dry eye symptoms by improving signs in a timely manner remains an unmet medical need and a relevant factor for patient compliance and treatment success.
FURTHER INFORMATION
-
DEWS II has redefined dry eye as a multifactorial disease of the ocular surface characterised by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play aetiological roles.
-
New diagnostic techniques and treatment options provide an opportunity for better patient management.
-
A directed treatment plan is recommended based on a four-stage differential diagnosis and relative severity of the condition to improve patient outcomes.
-
Multiple factors contribute to dry eye and developments remain focused on longer-lasting therapies requiring less frequent dosing.
-
Instant relief of dry eye symptoms by improving signs in a timely manner remains an unmet medical need and a relevant factor for patient compliance and treatment success.
References
1. Market Scope 2016 Dry Eye Products Report: A Global Market Analysis for 2015 to 2021.
2. Vehof J, Hammond CJ. Incidence, persistence and resolution of dry eye disease: new insights into the natural history. Presentation at ARVO 2017 annual meeting, Baltimore, USA; May 7-11 2017.
3. Fernandez K, Ying G-S, Masssaro-Giordano G, et al. A survey of dry eye practice patterns. Presentation at ARVO 2017 annual meeting, Baltimore, USA; May 7-11 2017.
4. Downie LE, Rumney N, Gad A, et al. Comparing self-reported optometric dry eye clinical practices in Australia and the United Kingdom: is there scope for practice improvement? Ophthalmic Physiol Opt 2016;36(2):140-51.
5. Quintana CL, Szczesna-Iskander DH, Iskander R. Automated non-invasive method for dry eye prediction. Presentation at ARVO 2017 annual meeting, Baltimore, USA; May 7-11 2017.
6. Milner MS, Beckman KA, Luchs JI, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders - new strategies for diagnosis and treatment. Curr Opin Ophthalmol 2017;27 Suppl 1:3-47.
7. Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev 2016;2:CD009729.
8. Ervin AM, Law A, Pucker AD. Punctal occlusion for dry eye syndrome. Cochrane Database Syst Rev 2017;6:CD006775.
9. Chiambaretta F, Doan S, Labetoulle M, et al; HA-trehalose Study Group. A randomized, controlled study of the efficacy and safety of a new eyedrop formulation for moderate to severe dry eye syndrome. Eur J Ophthalmol 2017;27(1):1-9.
10. Labetoulle M, Chiambaretta F, Shirlaw A, et al. Osmoprotectants, carboxymethylcellulose and hyaluronic acid multi-ingredient eye drop: a randomised controlled trial in moderate to severe dry eye. Eye (Lond) 2017 [Epub ahead of print]
11. Baudouin C, Figueiredo FC, Messmer EM, et al. A randomized study of the efficacy and safety of 0.1% cyclosporine A cationic emulsion in treatment of moderate to severe dry eye. Eur J Ophthalmol 2017 [Epub ahead of print].
12. Godin MR, Gupta PK. Lifitegrast ophthalmic solution in the treatment of signs and symptoms of dry eye disease: design, development, and place in therapy. Clin Ophthalmol 2017;11:951-7.
13. Robert PY, Cochener B, Amrane M, et al. Efficacy and safety of a cationic emulsion in the treatment of moderate to severe dry eye disease: a randomized controlled study. Eur J Ophthalmol 2016;26(6):546-55.
14. Zhang X, M VJ, Qu Y, et al. Dry Eye Management: Targeting the ocular surface microenvironment. Int J Mol Sci 2017;18(7).pii:E1398.
15. Roy NS, Wei Y, Kuklinski E, Asbell PA. The growing need for validated biomarkers and endpoints for dry eye clinical research. Invest Ophthalmol Vis Sci 2017;58(6):BIO1-BIO19.
16. Praestegaard M, Gomez F, Heegaard SK, Dart JK. Retrospective evaluation of real-life efficacy of PADciclo™ 0.06% ciclosporin in dry eye patients. Presentation at ARVO 2017 annual meeting, Baltimore, USA; May 7-11 2017.
17. Lambiase A, Sullivan BD, Schmidt TA, et al. A two-week, randomized, double-masked study to evaluate safety and efficacy of lubricin (150 μg/ml) eye drops versus sodium hyaluronate (Ha) 0.18% eye drops (Vismed®) in patients with moderate dry eye disease. Ocul Surf 2017;15(1):77-87.
COMMENTS ARE WELCOME