The goal of glaucoma management is to prevent visual loss and disease progression in the patient’s lifetime through effective lowering of intraocular pressure (IOP), the primary modifiable risk factor in glaucoma. Sustained and consistent IOP reduction is key to halting the potentially damaging effects of progressive glaucoma. Most glaucoma blindness is avoidable, dependent however on early detection, tailored pressure-lowering treatment, sound persistence and regular progression monitoring.
Mainstay recommendations
The European Glaucoma Society (EGS) defines the aim of glaucoma treatment as preservation of adequate vision and related quality of life, with minimal or no side-effects, no disruption of normal activities and at sustainable cost [1]. Clinical guidelines from the UK National Institute for Health and Care Excellence (NICE) recommend the following for confirmed early or moderate chronic open-angle glaucoma (OAG), ocular hypertension and glaucoma suspects and advanced chronic OAG [2]:
- Initiate treatment with a prostaglandin analogue for individuals newly diagnosed with early or moderate chronic OAG and at risk of significant visual loss in their lifetime. More than one antiglaucoma medication may be needed concurrently to achieve target IOP.
- Hypotensive antiglaucoma treatment should be offered to individuals with ocular hypertension and suspected chronic OAG based on estimated risk of conversion to chronic OAG using IOP, central corneal thickness (CCT) and age, i.e. treat where IOP is greater than 25mmHg and central corneal thickness is 590 micormetres or less. High risk of conversion to chronic OAG is defined as IOP more than 25 and up to 32mmHg and CCT less than 555 micrometres, or IOP more than 32mmHg.
- Surgery with pharmacological augmentation (mitomycin C or 5-fluorouracil) as indicated should be offered to individuals with advanced chronic OAG or considered at risk of progressive sight loss despite treatment.
Target IOP refers to the IOP level that prevents further glaucoma damage or an IOP level that reduces the risk of significant visual loss during the patient’s lifetime. Key factors in considering an appropriate target IOP include extent of pre-existing damage and rate of progression. Other factors are pressure level at which damage occurred, CCT, age and life expectancy, family history, race and costs / risk of treatment or lack of treatment. The term ‘maximal-tolerable medical therapy’ is considered of limited usefulness. As Goyal and Barton note, there is little evidence of increasing benefit by adding more than two or three glaucoma drops to an antiglaucoma medical regimen, and intolerance rises substantially with the use of four to five drugs [3].
Broad selection of glaucoma drops available
Many patients with glaucoma or at high risk of conversion to glaucoma are effectively controlled on topical glaucoma medication. Prostaglandin-based antiglaucoma drops are the most commonly prescribed drug class in glaucoma, providing effective first-line medical therapy in the majority of glaucoma patients.
A systematic analysis evaluating the pressure-lowering effects and tolerability of prostaglandin analogues found the greatest pressure lowering effect (mean IOP change from baseline) with bimatoprost, although the incidence of hyperaemia was lower with latanoprost and travoprost [4]. Results from an eight-week randomised parallel group study, involving newly diagnosed glaucoma patients, found that latanoprost, bimatoprost and travoprost produced comparable IOP reductions that were indistinguishable within statistical parameters [5].
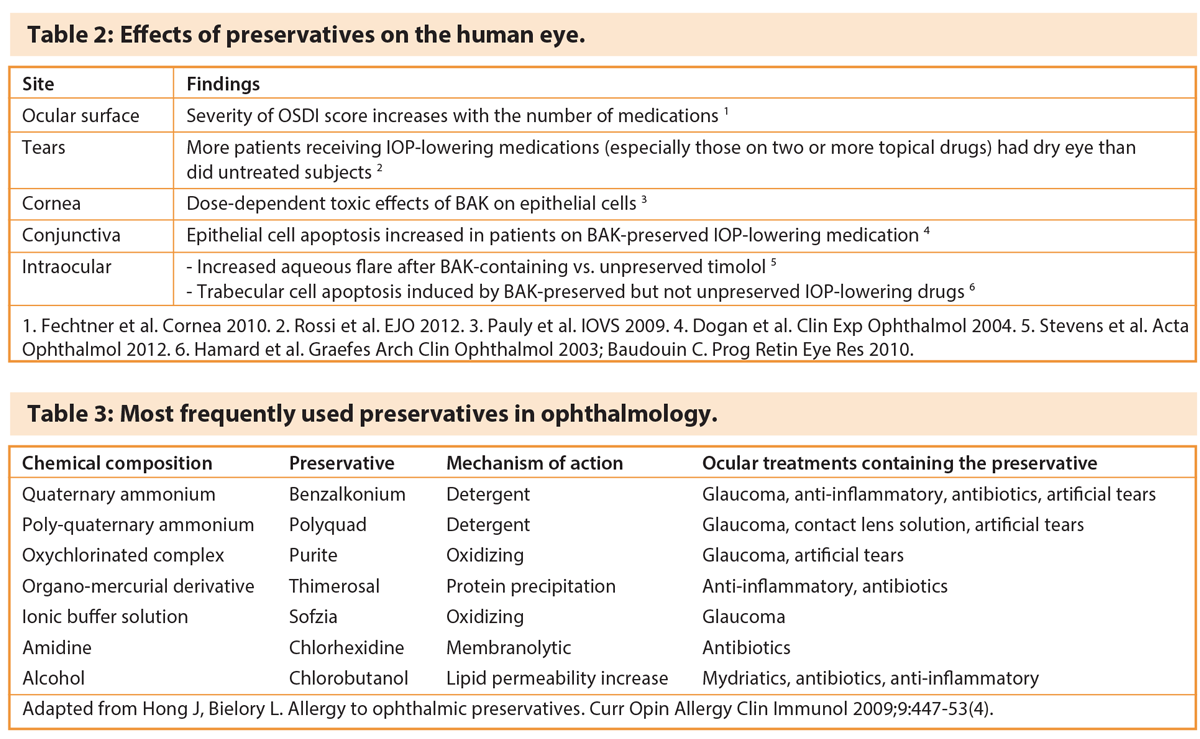
Where pressure targets are not achieved by initial monotherapy, additional ocular hypotensive medications may be used as second-line therapies, either in addition to first-line prostaglandin therapy or substituted if first-choice therapy is ineffective or poorly tolerated. These include ß-blockers, carbonic anhydrase inhibitors and alpha-agonists (Table 1).
Fixed-combination antiglaucoma formulations help improve adherence, simplifying dose regimen and offering superior tolerability with reduced exposure to excipients and preservatives. Combination agents provide equivalent or improved efficacy and safety compared to concomitant use of individual components, helping avoid a washout effect where a second drop may reduce the effect of the first [1]. Studies show that the fixed combination of bimatoprost and timolol was more effective than bimatoprost in patients whose pressure was not controlled with prostaglandin eye drops alone [6,7]. This once-daily fixed combination drop lowered IOP to less than 18mmHg in 18.7% of these patients compared with 10.2% with bimatoprost alone. Also, more patients given fixed combination bimatoprost and timolol had a pressure reduction exceeding 20% (67.9% against 48.9%).
Compliance and persistence with glaucoma drops is notoriously poor. But, as the EGS notes, fixed-combination eye drops provide the potential for improved patient compliance and less side-effects due to a reduced level of preservatives. Combined preparations have also been shown to result in less ocular side-effects. Practitioners should exclude contraindications to ß-blockers when prescribing fixed-combination drugs containing a ß-blocker.
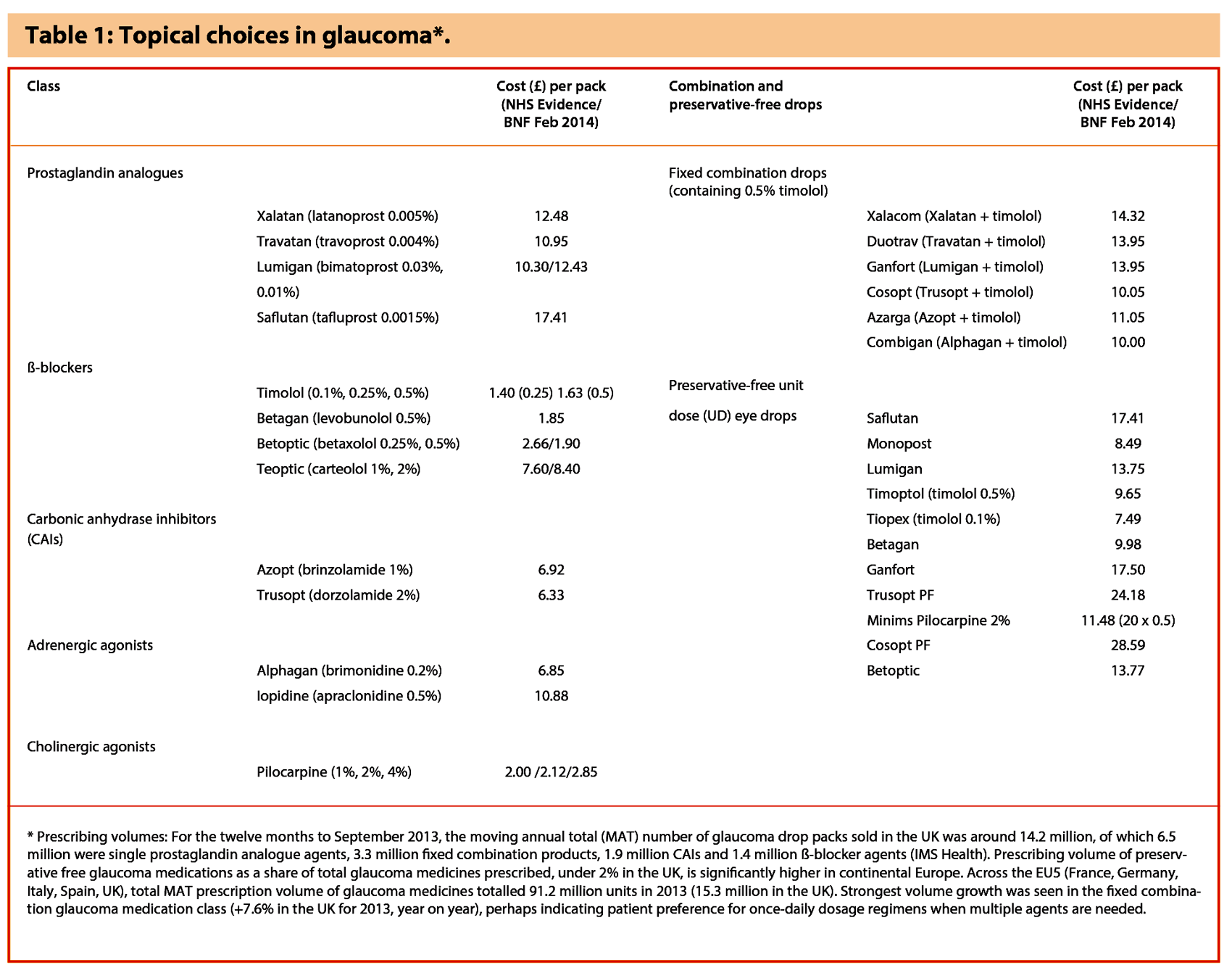
Preservatives are usually added to eye drop formulations to prevent microbial contamination (Table 2). Due to potential toxicity, practitioners are encouraged to consider the use of preservative-free glaucoma medications in appropriate patient populations, especially those with ocular surface disease.
Ocular surface disease and the benefit of preservative-free glaucoma drops
The European Medicines Agency, in a statement on antimicrobial preservatives in ophthalmic preparations, recommends formulations without preservatives for those patients who do not tolerate eye drops with preservatives [8]. Ophthalmic preparations without preservatives are strongly recommended for use in paediatric patients. Where preservatives are needed, the concentration should be kept at the minimum level consistent with satisfactory antimicrobial function (Table 3).
An evaluation to determine the incidence of ocular toxicity of preservatives with glaucoma medications found that ocular symptoms and signs were less prevalent when preservative-free drops were used [9]. Other data show that a large proportion of patients with OAG or ocular hypertension have signs and / or symptoms of ocular surface disease in at least one eye. Coexistence of ocular surface disease and use of eye drops containing benzalkonium chloride (BAK), the most widely used preservative, may adversely impact vision-related quality of life [10].
Ocular surface disease is more common in patients with increasing glaucoma severity and is associated with poorer glaucoma-related quality of life and higher daily exposure to BAK [11]. Benzalkonium chloride, a cationic surfactant, even in doses of 0.002% to 0.004%, can result in toxic effects on the surface of the eye and ocular inflammation. Overall, around 5-6% of all treated glaucoma patients experience some form of preservative sensitivity leading to discontinuation of preserved ophthalmic solutions in practice. It’s also likely that there is reduced adherence to treatment when treatment-related side-effects are experienced, increasing the risk of disease progression through uncontrolled IOP.
Up to one in five patients on topical treatment for glaucoma or ocular hypertension may require preservative-free antiglaucoma treatment [12]. Patient populations that may benefit most from preservative-free topical glaucoma medication include: clinically relevant dry eye disease (stage 2); in need of concomitant topical therapy; long life expectancy; blepharitis or meibomian gland dysfunction; intolerance to the preservative; and high risk of need for surgical intervention [12]. Long-term application of BAK increases the risk of filtration surgery failure, which has been linked to the subclinical conjunctival inflammation induced by ophthalmic solutions.
Baudouin et al. advise the use of BAK-free solutions whenever possible, especially in patients with the greatest exposure to high doses or prolonged treatments, in those with pre-existing or concomitant ocular surface disease and those experiencing side-effects related to ocular surface [13]. Exposure to BAK can be reduced, without compromising IOP control, by using preservative-free preparations, BAK-free formulations or fixed combinations to reduce the number of drops.
A recently published meta-analysis assessing the relative efficacy and tolerability of preservative-free latanoprost compared with other prostaglandin analogues reported that preservative-free latanoprost was non-inferior to other prostaglandin analogues in terms of pressure lowering efficacy [14]. The analysis also found that hyperaemia rates were statistically significantly lower with preservative-free latanoprost. Ganfort single dose (UD) formulation represents the first prostaglandin fixed combination glaucoma treatment for patients in the UK with OAG or ocular hypertension who require a preservative-free formulation. This once-daily preservative-free fixed combination drop provides equivalent efficacy to the multi-dose formulation; also, it does not require refrigeration and patients can choose to instil the drop in the morning or evening.
Clinicians emphasise that it is important to identify which individuals would benefit from preservative-free antiglaucoma drops through careful clinical evaluation of the individual patient. However, while the toxic effects of topical preservatives are well described, not all patients are affected. Other treatment considerations include efficacy, nonadherence, ease of use, education and quality of life.
Indications for surgery
A relatively small percentage of patients (~5%) with chronic open-angle glaucoma require glaucoma surgery.
Potential indications for glaucoma surgery include:
- inadequate IOP control
- progression of visual fields despite seemingly good IOP control
- intolerable side-effects of treatment
- advanced glaucoma at presentation (fixation-threatening visual field defects)
- problems with drop adherence
- lack of availability and inability to afford costs of glaucoma medication
- patient’s preference for surgery
Glaucoma surgery is usually considered if an individual presents with advanced glaucoma or if there is glaucomatous progression despite medical therapy with two to three classes of hypotensive drugs or after laser trabeculoplasty [3]. Patients should be offered surgery in cases of inadequate IOP control on two drops or advanced glaucoma (MD≤12dB or sensitivity <15dB in the central degrees in both hemifields) at presentation, as these patients are at highest risk of loss of central vision from glaucoma [2].
First surgery appears to provide the best chance of success. In addition, data show that the degree of IOP reduction after trabeculectomy – and subsequent IOP variability – may play a key role in the progression of glaucoma. Kotecha et al. from the MoreFlow Study Group found that approximately one third of eyes continued to show progression of glaucoma at five years after trabeculectomy [15]. These typically had significantly higher median IOP over follow-up compared with non-progressing eyes (16.5mmHg compared with 14mmHg) and less IOP reduction from baseline values (23% vs. 39% median reduction over the follow-up period).
Keeping an eye on progression
The lifetime risk of blindness or visual disability is not negligible in patients with glaucoma [16]. A small but significant proportion of all glaucoma patients under clinical care are estimated to be at risk of severe visual field impairment during their predicted lifetime. This risk increases significantly with more advanced visual field loss, increasing lifetime and a high progression rate.
Measuring rates of visual field change in glaucoma requires frequent visual field examinations. Three examinations each year are recommended to identify an overall change in mean deviation of 4dB over two years in a patient with average visual field variability [17]. Practitioners should measure the rate (speed) of VF progression, perform sufficient visual field examinations to detect change and increase frequency of testing after diagnosis.
A retrospective study using VF series of more than three years’ duration from 3790 UK patients in glaucoma clinics, designed to characterise rates of VF loss, found that 3% (95% confidence interval 2.7%-3.4%) of patient eyes progressed at faster than -1.5 dB/year [18]. For patients with both eyes followed, 5.2% (CI 4.5%-6.0%) were predicted to progress to statutory blindness, with a further 10.4% reaching visual impairment in their lifetime. This study and other evidence underscore the need to reliably detect VF defects early before they become moderately or severely damaged.
However, there remains wide variation in attitudes to follow-up intervals for glaucoma patients. A survey of real-world practice amongst UK glaucoma specialists found that nearly half (46%) agreed that six visual field tests in the first two years was ideal practice, while 28% said this practice was ‘not possible’, citing resource constraints [19].
Optic disc progression can be reliably confirmed by robust stereoscopic disc photographs. Digital stereo optic disc images are useful for evaluating the optic disc in glaucoma and allow the application of advanced imaging processing applications. Stereo disc photography also provides a reliable archive to allow meaningful comparison over the long term, without over reliance on changeable software suites. Chauhan and Burgoyne emphasise monitoring of structural changes, involving incorporation of spectral-domain ocular coherence tomography (OCT) imaging of the optic nerve head into the clinical examination of the optic disc [20].
Glaucoma is real
Glaucoma typically is a chronic asymptomatic disease, but it’s a real disease that can cause harm if unchecked. Most individuals with chronic OAG are effectively managed with topical medical therapy, and the introduction of preservative-free glaucoma therapy is a welcome step forward in promoting compliance and persistence in patients with ocular surface disease or those on prolonged multiple treatment regimens. That said, patients – treated with drops, laser and / or surgery – might still progress despite apparently sound control of IOP. Practitioners are encouraged to look out for progressive glaucoma and be aware of those at risk of bilateral blindness, particularly in the young, those with severe disease, non-compliant patients and other high-risk individuals.
References
1. The European Glaucoma Society. Terminology and guidelines for glaucoma (3rd Edition). The European Glaucoma Society 2008:
www.eugs.org/eng/EGS_guidelines.asp.
2. National Institute for Health and Care Excellence. Glaucoma: diagnosis and management of chronic open angle glaucoma and ocular hypertension. NICE clinical guideline 85, issued April 2009.
3. Goyal S, Barton K. Chapter 34: Indications for surgery. In Spaeth GL, Danesh-Meyer, Goldberg I, Kampik A (Eds). Ophthalmic Surgery: Princples and Practice. Elsevier Health Sciences; 2011.
4. Aptel F, Cucherat M, Denis P. Efficacy and tolerability of prostaglandin analogs: a meta-analysis of randomized controlled clinical trials. J Glaucoma 2008;17(8):667-73.
5. Yildirim N, Sahin A, Gultekin S. The effect of latanoprost, bimatoprost, and travoprost on circadian variation of intraocular pressure in patients with open-angle glaucoma. J Glaucoma 2008;17(1):36-9.
6. European Medicines Agency. Ganfort (bimatoprost/timolol). EPAR summary for the public. EMA/684020/2012, EMEA/H/C/000668.
7. Ganfort (bimatoprost/timolol ophthalmic solution 0.03% / 0.5% in a single dose container). Summary of Product Characteristics.
8. European Medicines Agency. EMEA public statement on antimicrobial preservatives in ophthalmic preparations for human use. London, 8 December 2009. EMEA/622721/2009.
9. Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol 2002;86(4):418-23.
10. Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma 2008;17(5):350-5.
11. Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol 2012;153(1):1-9.
12. Stalmans I, Sunaric Mégevand G, Cordeiro MF, et al. Preservative-free treatment in glaucoma: who, when, and why. Eur J Ophthalmol 2013;23(4):518-25.
13. Baudouin C1, Labbé A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res 2010;29(4):312-34.
14. Cucherat M, Stalmans I, Rouland JF. Relative efficacy and safety of preservative-free latanoprost (T2345) for the treatment of open-angle glaucoma and ocular hypertension: an adjusted indirect comparison meta-analysis of randomized clinical trials. J Glaucoma 2014;23(1):e69-75.
15. Kotecha A, Spratt A, Bunce C, et al; MoreFlow Study Group. Optic disc and visual field changes after trabeculectomy. Invest Ophthalmol Vis Sci 2009;50(10):4693-9.
16. Cedrone C, Nucci C, Scuderi G, et al. Prevalence of blindness and low vision in an Italian population: a comparison with other European studies. Eye (Lond) 2006;20(6):661-7.
17. Chauhan BC, Garway-Heath DF, Goñi FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol 2008;92(4):569-73.
18. Saunders LJ, Russell RA, Kirwan JF, et al. Examining visual field loss in patients in glaucoma clinics during their predicted remaining lifetime. Invest Ophthalmol Vis Sci 2014;55(1):102-9.
19. Malik R, Baker H, Russell RA, Crabb DP. A survey of attitudes of glaucoma subspecialists in England and Wales to visual field test intervals in relation to NICE guidelines. BMJ Open 2013;3(5).
20. Chauhan BC, Burgoyne CF. From clinical examination of the optic disc to clinical assessment of the optic nerve head: a paradigm change. Am J Ophthalmol 2013;156(2):218-27.
TAKE HOME MESSAGE
-
Most glaucoma blindness is avoidable, but is dependent on early detection, tailored pressure-lowering treatment and regular progression monitoring.
-
To improve compliance and persistence, consider using preservative-free topical glaucoma medicines in patients with ocular surface disease or those on prolonged multiple dose regimens.
-
Glaucoma surgery is indicated in cases of advanced glaucoma or if there is glaucomatous progression despite maximal medical therapy (two or three classes of hypotensive drugs).
-
A small but significant proportion of all glaucoma patients under clinical care are estimated to be at risk of severe visual field impairment during their predicted lifetime.
-
Evidence underscores the need to reliably detect visual field defects early before they become moderately or severely damaged.
COMMENTS ARE WELCOME