The incidence of Idiopathic intracranial hypertension (IIH) is increasing, likely in line with the worldwide epidemic of obesity. To date, there have been revisions in the terminology used and diagnostic criteria for IIH; these recognise the need to exclude secondary causes of increased intracranial pressure. Recently published randomised controlled trials have investigated effect of weight loss and use of acetazolamide in IIH.
Although various treatment options are currently used, the current Cochrane review highlighted a lack of randomised controlled studies to fully support medical treatment in IIH [1]. Currently in the UK there are trials exploring potential treatments for IIH including bariatric surgery. This ongoing research has the potential to increase our understanding of the aetiology of the disease and improve standards of care for our IIH patients. This article reviews the developments to date and their implication for current clinical practice.
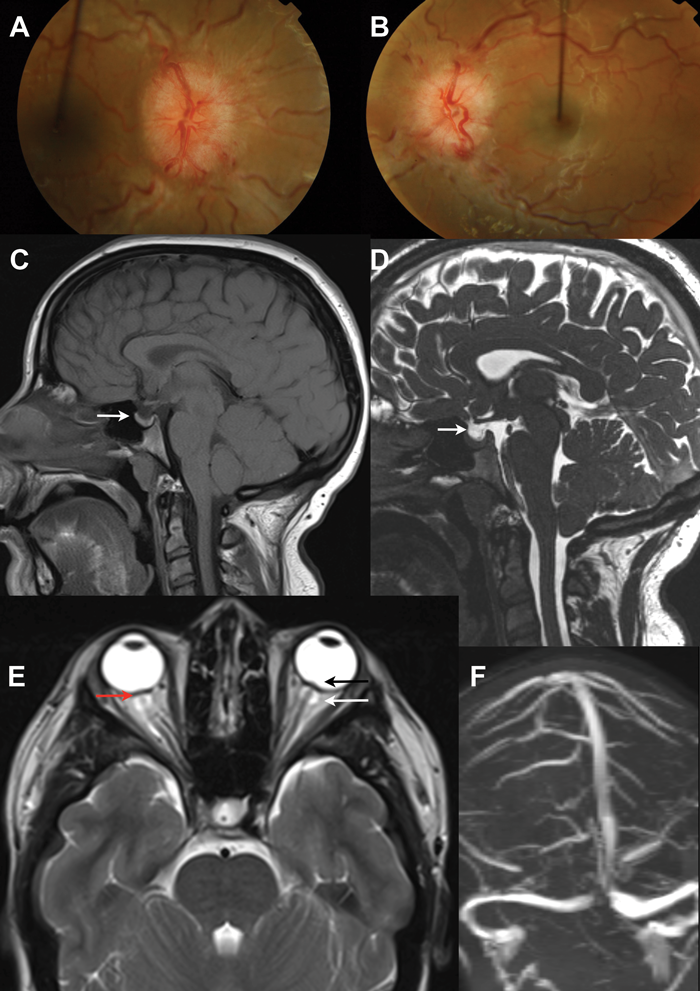
IIH occurs predominantly in women and although the underlying pathogenesis is not fully understood, it has a prominent association with obesity [2]. The condition is well known to ophthalmologists as the majority of patients typically present from their opticians or directly to the eye casualty with headaches and papilloedema (Figures 1A and B). Other symptoms may include blurred vision, visual obscurations, pulsatile tinnitus and double vision: none of which are pathognomonic for IIH [3]. The papilloedema is investigated urgently with neuro-imaging (Figures 1C-F) and lumbar puncture (LP) [4]; where there is raised LP opening pressure and if no underlying cause is found the patient is diagnosed with IIH [5].
Epidemiology
IIH is recognised to typically affect obese young women with a previous annual incidence of approximately one per 100,000 (1990-2001) in the general population, with an increase to 2.4 per 100,000 (2002-2014; P = 0.007) [6]. Male patients account for 10% of IIH cases and only 5% are over 50-years-old.
“The principles of management of IIH are to treat the underlying disease, protect the vision and reduce the headache morbidity.”
Current terminology
There have been recent developments in terminology used and diagnostic criteria for IIH, these recognise the need to exclude secondary causes of increased intracranial pressure (ICP) and have helped standardise the recruitment to clinical trials in IIH. Using the term ‘benign intracranial hypertension’ (BIH) has been suggested to be inappropriate as although some patients may follow a mild and self-limiting course, for others the morbidity from visual loss and persistent headache is far from benign. The term ‘pseudotumor cerebri’ (PTC) describes the clinical syndrome resulting from elevated ICP but includes those with a secondary cause that are therefore not ‘idiopathic’. IIH is characterised as patients with raised ICP of unknown aetiology fulfilling the criteria set out in the revised modified Dandy criteria [7].
Figure 1:
1A and 1B show colour fundus photos of bilateral disc swelling secondary to raised intracranial pressure (papilloedema).
The neuro-imaging features are non-specific and not diagnostic of IIH:
1C and 1D show MRI T2 and T1 sagittal images showing a partially empty sella.
1E is MRI showing distension of optic nerve sheaths (white arrow), flattening of the posterior globes (red arrow) and optic nerve head protrusion (black arrow).
IF is MR venography showing no cerebral sinus thrombosis.
Updating the diagnostic criteria
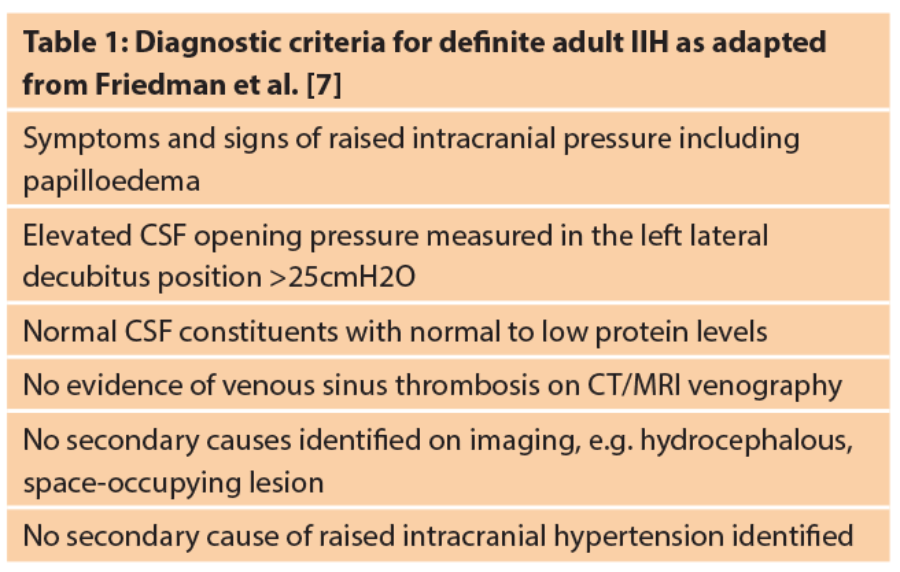
The diagnostic criteria for IIH have evolved but retain the framework of the original criteria proposed by Dandy in 1937, through the modified Dandy criteria, to the updated modified Dandy criteria (Table 1) [7]. These revised criteria emphasise the need to exclude secondary causes of elevated ICP and the need for reliable cerebrospinal fluid (CSF) pressure measurement. An accurate diagnosis of IIH is dependent on neuroimaging that excludes space occupation, hydrocephalus and cerebral venous sinus thrombosis. There are several neuroimaging changes that are associated with raised ICP including empty sella (Figures 1C and D), distension of optic nerve sheaths (Figure 1E, white arrow), vertical tortuosity of the optic nerves and flattening of the posterior globes (1E, red arrow) and protrusion of the optic nerve head (Figure 1E, black arrow), but all of these are non-specific and not diagnostic of IIH.
Diagnosis is also dependent on a CSF opening pressure of >25cm CSF performed in the later decubitus position. CSF of 10-25cm represents the 95% reference interval for opening pressure in normal subjects and opening pressures of 30cm CSF or higher may be observed in normal subjects. CSF opening pressure can be influenced by body position, therefore the lateral decubitus position is recommended. The neuroimaging (Figures 1D-F) and CSF opening pressure specifications in the revised modified Dandy criteria should improve diagnostic accuracy and standardise recruitment for clinical trials in IIH.
Evidence for disease modifying therapy in patients with IIH
The principles of management of IIH are to treat the underlying disease through weight management, protect the vision and reduce the headache morbidity. Due to the overwhelming association of IIH with obesity, weight loss is frequently recommended as a treatment for IIH. The first study to use diet to treat IIH was reported in 1975. In this uncontrolled prospective case series, nine patients were treated with a low calorie rice diet (with fluid and sodium restriction); the resultant weight loss (13-38%) was associated with improvement in IIH symptoms and subjective assessments of papilloedema [8]. Subsequently, a landmark study was published in the BMJ in 2010 which reported the disease modifying effects of weight loss in IIH. This two stage prospective cohort study evaluated 25 women with IIH and compared a baseline three month observation period with a three months low calorie diet (425kcal/day).
The study found significant improvement in ICP (mean 8cm CSF) associated with weight loss (mean 15.7kg). Significant reduction in headache impact score was reported along with improvement in papilloedema measures (optic disc elevation and optic nerve sheath diameter with ultrasound and peripapillary retinal thickness with optical coherence tomography); improvements were sustained at three months post diet. This study has established the first evidence that weight loss reduces ICP and was effective in improving headache disability, as well as reducing headache severity, frequency and analgesic use by at least 50% in 50% of patients [9]. Additionally, unlike other symptomatic treatment in IIH (analgesia, acetazolamide, CSF shunting) weight loss is the only treatment to modify the underlying disease process.
Long-term maintenance of weight loss is notoriously difficult and is typically as little as 2-4kg at two years, irrespective of the dietary regime followed [10]. In keeping with this, patients in the IIH weight loss study [9] were noted to regain weight in the year following discontinuation in the study and consequently their symptoms and signs of IIH relapsed, a documented phenomenon in IIH [11]. Consequently, alternative sustainable approaches to weight loss in IIH are likely to offer patients an effective, potentially curative treatment.
Recent evidence for symptomatic treatment in patients with IIH
The current Cochrane review on IIH management reported on the use of acetazolamide, a carbonic anhydrase inhibitor, in IIH [1]. To summarise, two trials were commented on:
- The largest trial to date is the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT) [12]; recruiting a total of 165 participants (86 patients in the acetazolamide group and 79 placebo control). The IIHTT investigated whether the use of acetazolamide in conjunction with a low-sodium weight-reduction diet compared with diet alone resulted in modest improvement in visual field function in patients with mild visual loss. The chosen primary outcome measure was the change in perimetric mean deviation (PMD) on Automated Visual Field Analyser at six months.
The study population included individuals with IIH and mild visual field loss, which was initially defined as PMD of -2 to -5dB in the worst eye, although this was expanded to include PMD of -2 to -7dB during the study to improve recruitment. The initial dosing was 500mg twice a day with a schedule to increase the dose by 250mg every six days up to a maximum of 4g daily. Participants who could not tolerate the study drug could decrease the dosage to a minimum of 125mg per day. Forty-four percent achieved the maximal dose of 4g daily, with the majority tolerating 1g/d (Hove, 2016). Both groups, aectazolamide with low sodium diet and placebo with low sodium diet, showed improvement in the primary outcome measure of PMD from baseline (-3.53dB in both groups). At six months the PMD in the acetazolamide group (-2.10dB) had improved more than the placebo group (-2.82dB) by a difference of 0.71dB (p=0.05). Side-effects of fatigue, nausea, diarrhoea, vomiting and paraesthesia were significantly higher in the acetazolamide group and more patients discontinued the study drug in the acetazolamide group than the placebo group. - The Birmingham study [13] recruited 50 participants (25 to acetazolamide and 25 to the placebo control). This feasibility study failed to show a treatment effect but importantly 48% discontinued acetazolamide due to adverse effects.
The Cochrane review concluded: “The two included RCTs showed modest benefits for acetazolamide for some outcomes, there is insufficient evidence to recommend or reject the efficacy of this intervention, or any other treatments currently available, for treating people with IIH” [1]. As a result of a lack of strong evidence in the setting of significant side-effects not all specialists treat IIH with acetazolamide.
Headache is a major morbidity in IIH [14], therefore, joint care between ophthalmology and neurology is essential for patients. Headache characteristics typically evolve as IIH progresses, and often when the papilloedema resolves the headache remains chronically. There is frequently a mixed phenotype of the headache in patients with IIH. Headache can be a combination of raised intracranial pressure, migraine and medication overuse headache [15,16]. There are currently no clinical trials in this area.
Role of the cerebral venous sinuses in IIH
All patients with papilloedema should be investigated with MR or CT venography to exclude cerebral venous sinus thrombosis (Figure 1F). Improvements in venography imaging studies now detail that many, if not all, with IIH have anatomical abnormalities of the cerebral venous sinus system. These include stenosis of the dominant or transverse sinus. What is not fully known is whether the stenosis result from intrinsic dural sinus anatomy or extrinsic compression by the increased intracranial pressure. Importantly, reducing ICP can lead to resolution of stenosis in most. The degree of stenosis does not appear to uniformly correlate with ICP or visual loss [17]. Neurovascular stenting has been reported, in a number of series, to lead to an improvement in symptoms and signs of intracranial hypertension.
Complications of the procedure include a short-lived ipslateral headache in many, stent-adjacent stenosis that require retreatment in a third, and in rare cases vessel perforation leading to acute subdural haematoma, stent migration and thrombosis. The majority of patients who undergo neurovascular stenting require long-term antithrombotic therapy, typically for a minimum of six months following neurovascular stenting treatment. Due to the controversy in this therapy the role of neurovascular stenting in IIH is not yet established and there is, however, a current controlled clinical trial recruiting in the United States [ClinicalTrials.gov Identifier NCT01407809].
Where to next with IIH?
The patient charity IIHUK is active and engaging with patients and clinicians. It has a useful website to signpost patients and families to, and runs meetings throughout the year in all parts of the UK (www.iih.org.uk). IIHUK is currently funding the James Lind Alliance (JLA) Priority Setting Partnership (PSP) for Adult IIH. JLA PSPs enable clinicians, patients and carers to work together to identify and prioritise uncertainties about the diagnosis and effects of treatments that could be answered by research. The JLA PSP for IIH is currently active and we would invite ALL ophthalmologists of ALL grades to contribute at http://www.iih.org.uk/index.php?
option=com_content&view=article&id=88
The IIH:Drug Trial
This was a Medical Research Council funded, phase 2 RCT based in Birmingham assessing the therapeutic efficacy and safety of an 11β-hydroxysteroid dehydrogenase type 1 inhibitor (AZD4017) to reduce ICP and treat IIH. The main outcome measure was lumbar puncture opening pressure at 12 weeks. This has completed recruitment and will likely report this autumn [ClinicalTrials.gov Identifier: NCT02017444].
The IIH:Weight Trial
This UK-based randomised controlled trial, funded by the National Institute for Health Research, aims to compare two methods of weight loss, bariatric surgery and a community dietary program, to investigate which offers the most effective long-term treatment for IIH. Bariatric surgery is recommended by the NICE clinical guidelines for patients with a Body Mass Index (BMI) of over 40, or over 35 with a co-morbidity. Women suffering from IIH have a BMI on average around 38 and IIH is not currently recognised as a co-morbidity for bariatric surgery. Recruitment is in the final stages. The study is expected to report in the autumn of 2018 [ClinicalTrials.gov Identifier: NCT02124486].
The Surgical IIH Trial
The Neuro-Ophthalmology Research Disease Investigator Consortium (NORDIC) have announced that they are currently planning a Surgical IIH Trial (SIIHT) comparing optic nerve sheath fenestration and CSF diversion in those with moderate to severe visual loss, with an outcome measure of perimetric mean deviation at six months. More details will be released soon (https://www.nordicclinicaltrials.com).
IIH:Labs is a translational research program evalauating pathogenics mechanisms and novel therapeutic targets in IIH using in vitro CSF secretion assay and in vivo models of ICP monitoring. Current projects are evaluating the steroid metabolite profile in IIH patients as well as efficacy of existing drugs used therapeutically to lower ICP in IIH.
These trials have the potential to increase our understanding of the aetiology of the disease; they may lead to novel future therapies; and help better inform our management decisions. As IIH involves clinicians from many specialties (neurology, ophthalmology, neurosurgery and neuroradiology), the Birmingham group have set up the UK CSF disorders day to facilitate discussion, research and education in IIH and other disorders of CSF disregulation. The next meeting is on Wednesday 27 September 2017 at the Bond Co. in central Birmingham. Professor Grant Liu (Neuro-Ophthalmology) will be visiting from Philadelphia and all ophthalmologists (of all grades) are most welcome. Please contact Susan Mollan or Alex Sinclair. Professor Grant Liu (Neuro-Ophthalmology) will be visiting from Philadelphia and all ophthalmologists (of all grades) are most welcome. Please see: https://www.qehb.org/shop/
product/uk-csf-disorder-day
TAKE HOME MESSAGE
-
The diagnostic criteria have been revised and will help reduce the diagnostic uncertainty in IIH.
-
Weight loss is currently the only disease modifying therapy in IIH.
Current trials using acetazolamide have shown not shown a clear enough benefit for all patients.
-
Headache is a major morbidity for those with chronic IIH, therefore referral or join care with neurology is essential.
-
The UK has a growing IIH research footprint and the JLA Patient Priority Setting will help establish the top 10 research questions in IIH (contribute now at www.iih.org.uk)



References
1. Piper RJ, Kalyvas AV, Young AMH, et al. Interventions for idiopathic intracranial hypertension. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No.: CD003434.
2. Markey KA, Mollan SP, Jensen R, Sinclair AJ. Understanding idiopathic intracranial hypertension: mechanisms, management, and future directions. Lancet Neurol 2016;15:78-91.
3. Yri HM, Jensen RH. Idiopathic intracranial hypertension: Clinical nosography and field-testing of the ICHD diagnostic criteria. A case-control study. Cephalalgia 2015;35(7):553-62.
4. Mollan SP, Markey KA, Benzimra JD, et al. A practical approach to, diagnosis, assessment and management of idiopathic intracranial hypertension. Pract Neurol 2014;14:380-90.
5. Mollan SP, Ali F, Hassan-Smith G, et al. Evolving evidence in adult idiopathic intracranial hypertension: pathophysiology and management. J Neurol Neurosurg Psychiatry 2016;87(9):982-92.
6. Kilgore KP, Lee MS, Leavitt JA, et al. Re-evaluating the incidence of idiopathic intracranial hypertension in an era of increasing obesity. Ophthalmology 2017;124(5):697-700.
7. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013;81:1159-65.
8. Newborg B. Pseudotumor cerebri treated by rice/reduction diet. Arch Intern Med 1974;133:802-7.
9. Sinclair AJ, Burdon MA, Nightingale PG, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ 2010;341:c2701.
10. Middleton KM, Patidar SM, Perri MG. The impact of extended care on the long-term maintenance of weight loss: a systematic review and meta-analysis. Obes Rev 2012;13(6):509-17.
11. Ko M, Chang SC, Ridha MA, et al. Weight gain and recurrence in idiopathic intracranial hypertension A case-control study. Neurology 2011;76(18):1564-7.
12. Wall M, McDermott MP, Kieburtz KD, et al. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA 2014;311(16):1641-51.
13. Ball AK, Sinclair AJ, Curnow SJ, et al. Elevated cerebrospinal fluid (CSF) leptin in idiopathic intracranial hypertension (IIH): evidence for hypothalamic leptin resistance? Clin Endocrinol (Oxf) 2009;70:863-9.
14. Mulla Y, Markey KA, Woolley RL, et al. Headache determines quality of life in idiopathic intracranial hypertension. J Headache Pain 2015;16:521.
15. Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology 2002;59(10):1492-5.
16. Menger RP, Connor DE, Jr, Thakur JD, et al. A comparison of lumboperitoneal and ventriculoperitoneal shunting for idiopathic intracranial hypertension: an analysis of economic impact and complications using the Nationwide Inpatient Sample. Neurosurg Focus 2014;37(5):E4.
17. Riggeal BD, Saindane AM, Ridha MA, et al. Clinical course of idiopathic intracranial hypertension with transverse sinus stenosis. Neurology 2013;80(3):289-95.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME