*Joint first authors
Keratoconus is a bilateral and asymmetric eye condition in which the cornea’s structure is affected and thinned, causing a cone-shaped bulge to develop. This results in progressive loss of vision and impairs the ability of the eye to focus [1]. The onset of the disease usually occurs in the second decade of life and its progression usually stabilises by the fourth decade.
Clinically, patients present with progressive irregular astigmatism, myopia, and ultimately vision impairment [2]. Paediatric keratoconus has an accelerated progression and can have an important negative impact on quality of life by affecting social and educational development of children [3]. Keratoconus has been described as the most common cornea ectatic disorder, but its epidemiology is highly variable worldwide [4].
Commonly presenting as a sporadic disorder, a minority of patients also have a family history of an autosomal dominant inheritance (6-24%) [4]. Some authors have reported a higher incidence of keratoconus in Indian, Pakistani, Middle Eastern, and Polynesian populations compared to Caucasian populations [5]. Additionally, younger age (<30 years old) and male gender have been identified as risk factors for keratoconus. Other risk factors include Down and Turner syndromes, Leber congenital amaurosis, as well as Marfan syndrome, Ehlers-Danlos, and osteogenesis imperfecta. The condition has also been associated with mitral valve prolapse, retinitis pigmentosa, ocular allergy, and atopic diseases. Chronic eye rubbing can favour the development of keratoconus and initiatives have been developed to increase awareness amongst patients against chronic mechanical manipulation [3,6].

Table 1: Signs and symptoms of keratoconus.
Clinical signs of keratoconus are diverse and depend on the progression of the disease at diagnosis (Table 1). Signs and symptoms can help identify keratoconus from its differential diagnoses including pellucid marginal degeneration, keratoglobus, post-refractive surgery ectasia, astigmatism, corneal scarring, and Terrien’s marginal degeneration [1].
Historically, keratoconus has been described in stages of early, moderate to advanced, and severe. The Amsler-Krumeich (AK) grading system has been most widely used and is based on keratometry and optical pachymetry, describing stages one to four based on spectacle correction, central keratometry, scarring, and central corneal thickness [7].
"Newer contact lens modalities have become available for conservative non-surgical management and surgical options such as collagen cross-linking with the option to combine with laser refractive procedures including topography guided ablation, INTACS, phakic IOLs and there have been further developments in techniques for corneal grafting"
Placido-based imaging, tomographic devices, and anterior segment ocular computerised tomography have all helped to increase the capability to image the anterior and posterior cornea, the latter being of significant value for diagnosis and treatment protocols in the current landscape. Advances in these have enabled much earlier detection of the condition, including in recent years the ability to screen for subclinical changes and thus helping direct earlier management and treatment with better prognosis and visual rehabilitation. In recent years the Berlin ABCD classification or staging system was developed on the Oculus Pentacam (Oculus GmbH, Wetzlar, Germany) and assists with progression analysis (Table 2) [8,9].
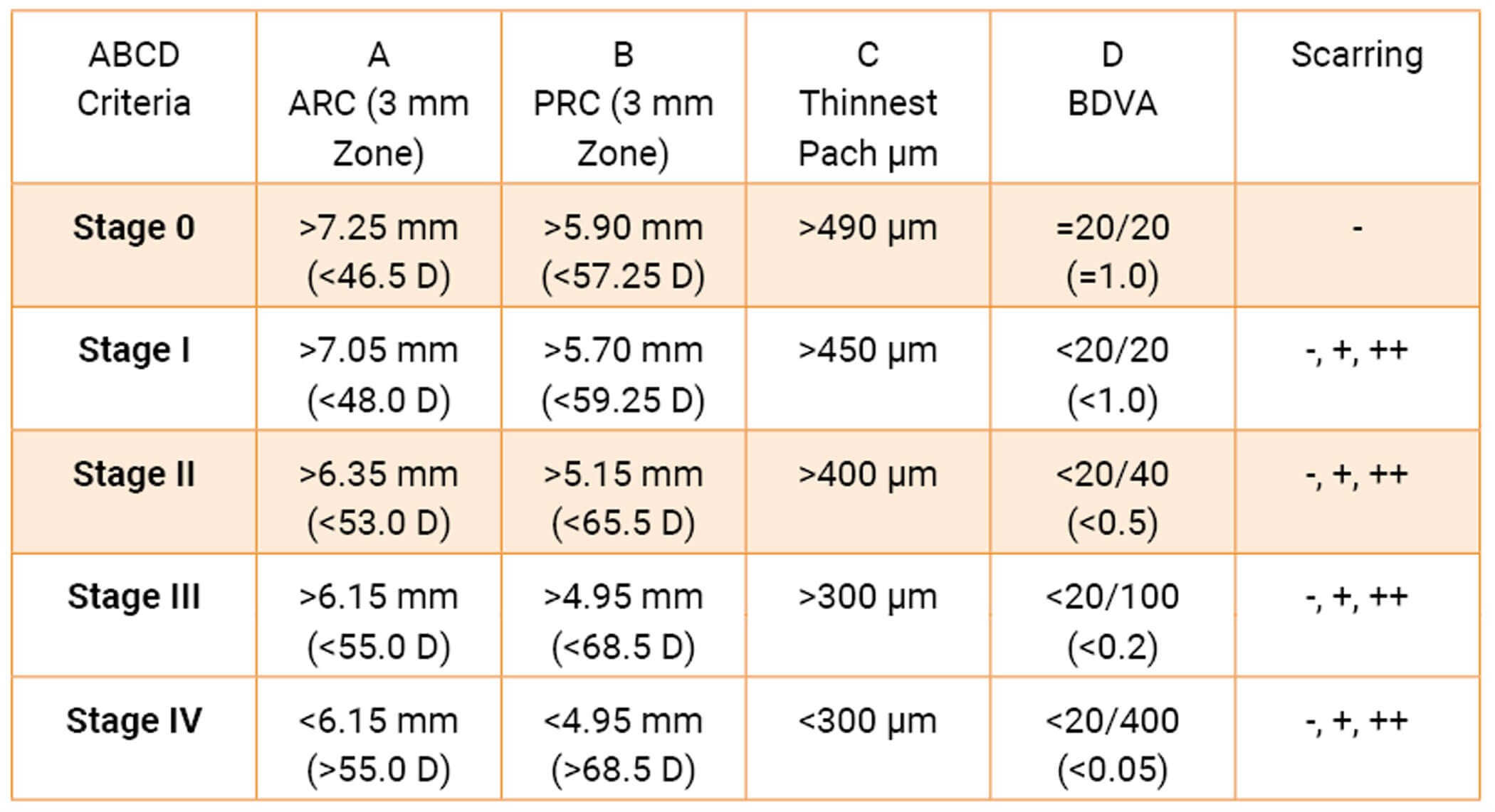
Table 2: Berlin ABCD system keratoconus staging system: This grading system includes the anterior radius of curvature (A), posterior radius of curvature (B), corneal pachymetry at thinnest (C) distance best-corrected vision (D) and scarring (none, that which does not obscure the iris details and that which does obscure the iris details).
In dynamic evolution, the diagnosis of keratoconus remains a challenge. The slit-lamp examination and corneal curve readings are usually used in clinical settings. Serial readings over time can be useful to monitor progression.
Traditionally, diagnosis was made by using the central corneal curvature and apical pachymetry readings. A keratometer, apical optical pachymeter, and subjective refractions were used for disease staging. However, central pachymetry was described by world expert consensus as the least reliable indicator for diagnosing keratoconus, because this condition can be present in a cornea of a normal central thickness. Paracentral thinning and bulging of the cornea and maximum thinning at the apex of protrusion could be seen. Although anterior changes in the cornea can be seen in symptomatic patients, this is usually a late finding and subclinical disease is often associated with changes in the posterior part of the cornea. In some patients, posterior corneal changes and / or changes in pachymetric distribution can be seen and are the first indicators of ectatic disease, despite a normal anterior curvature [10].
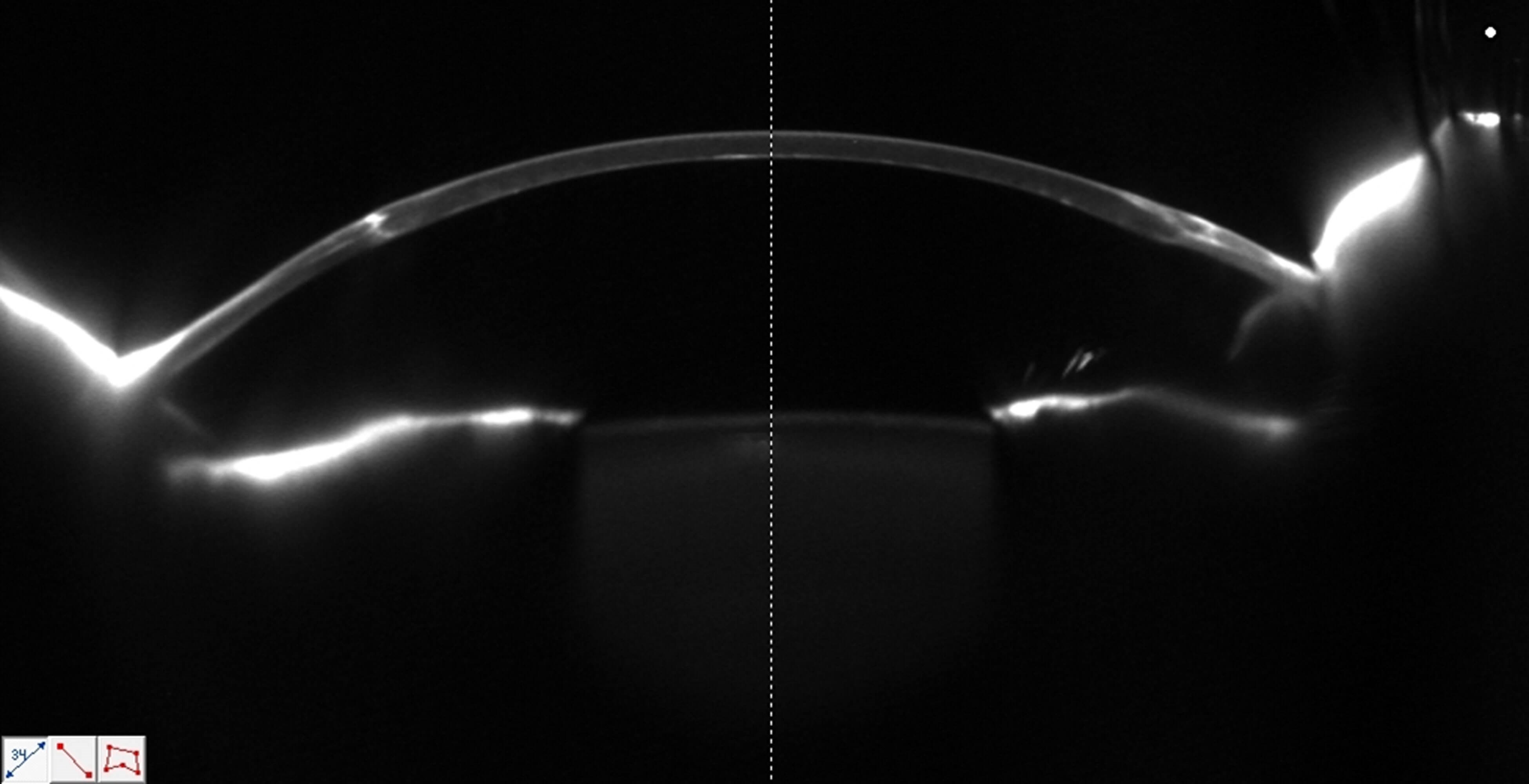
Schiempflug image of corneal graft.
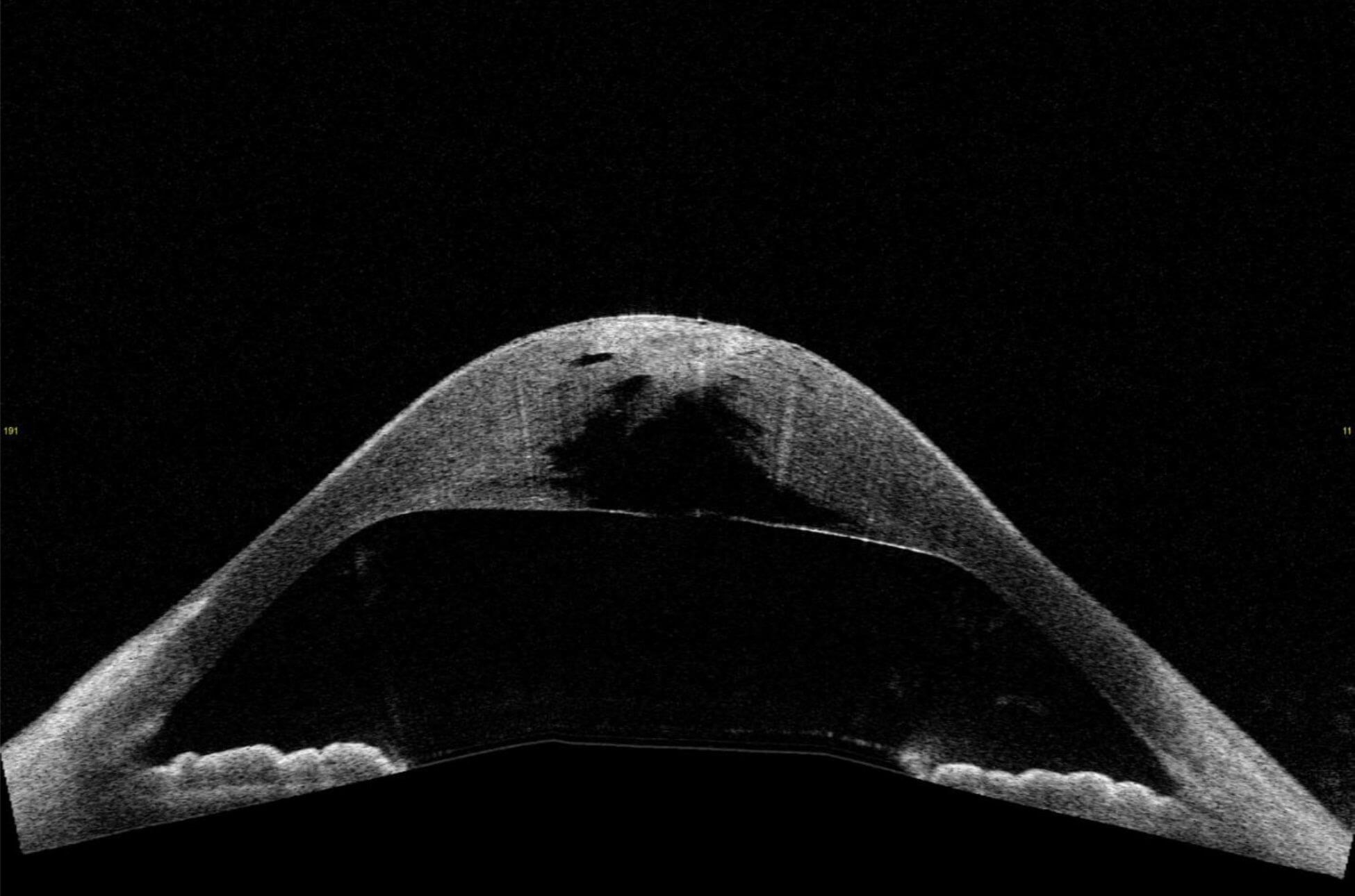
Anterior segment OCT of hydrops (complication of advanced keratoconus).
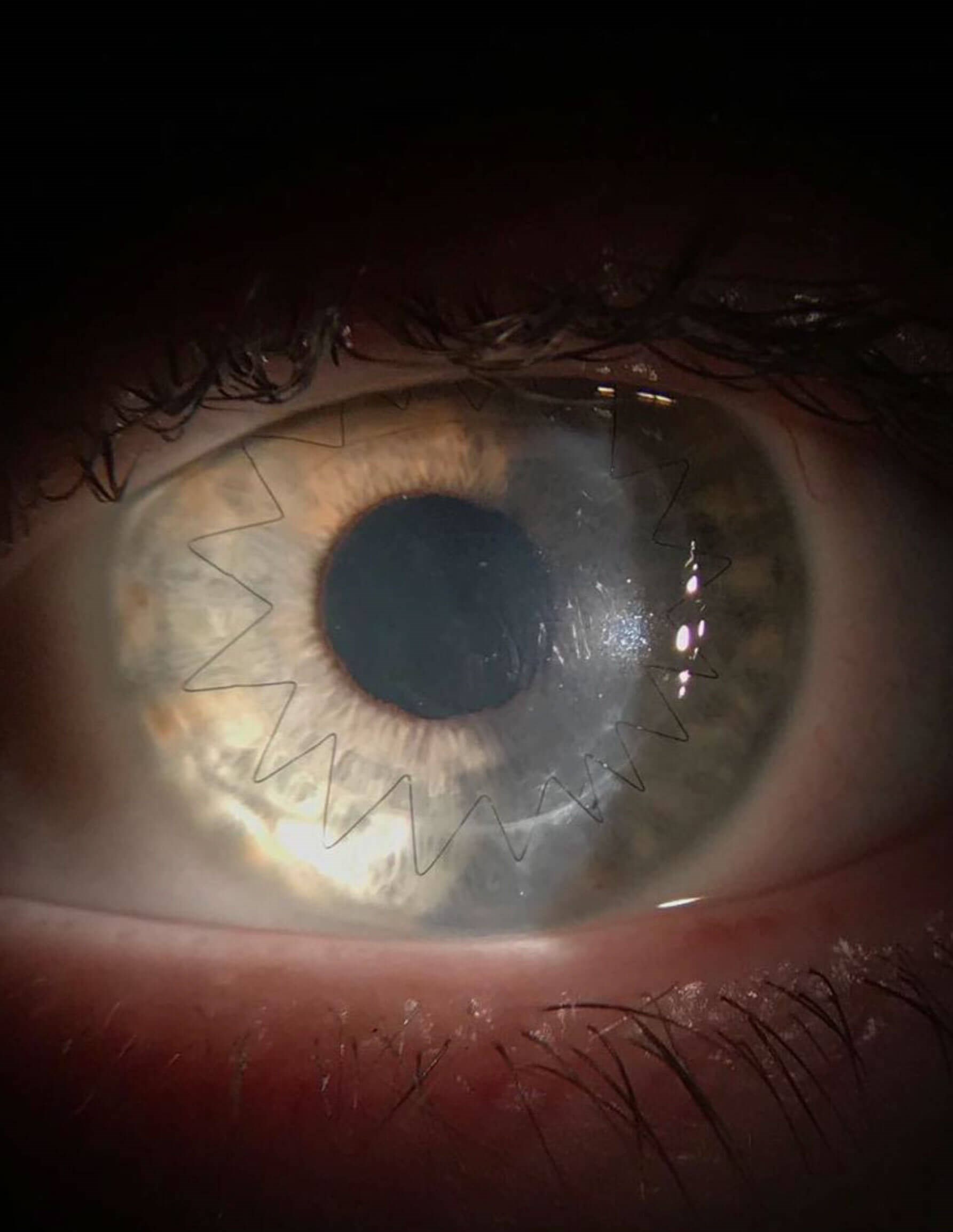
Slit-lamp microscope image of corneal graft.
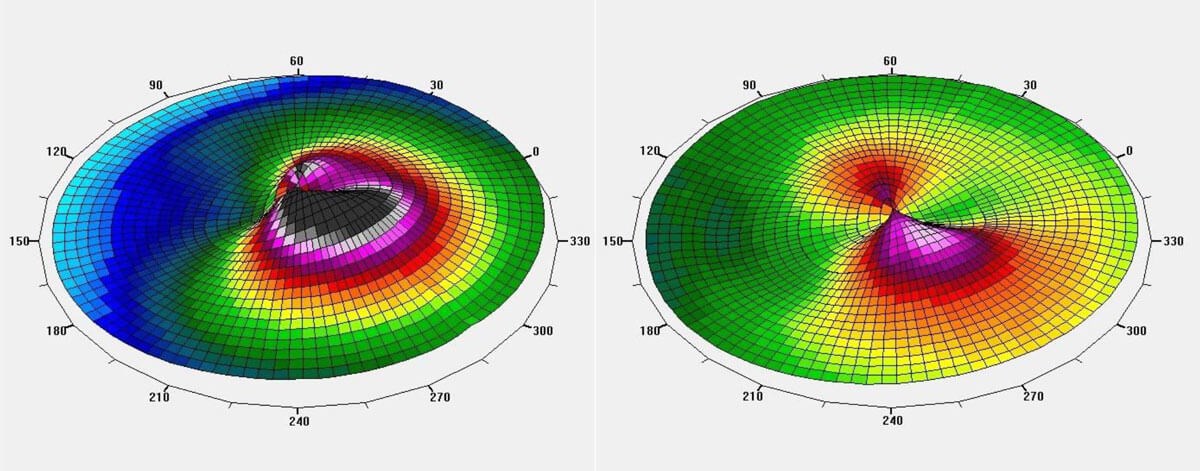
Topography-guided laser + CXL comparison maps.
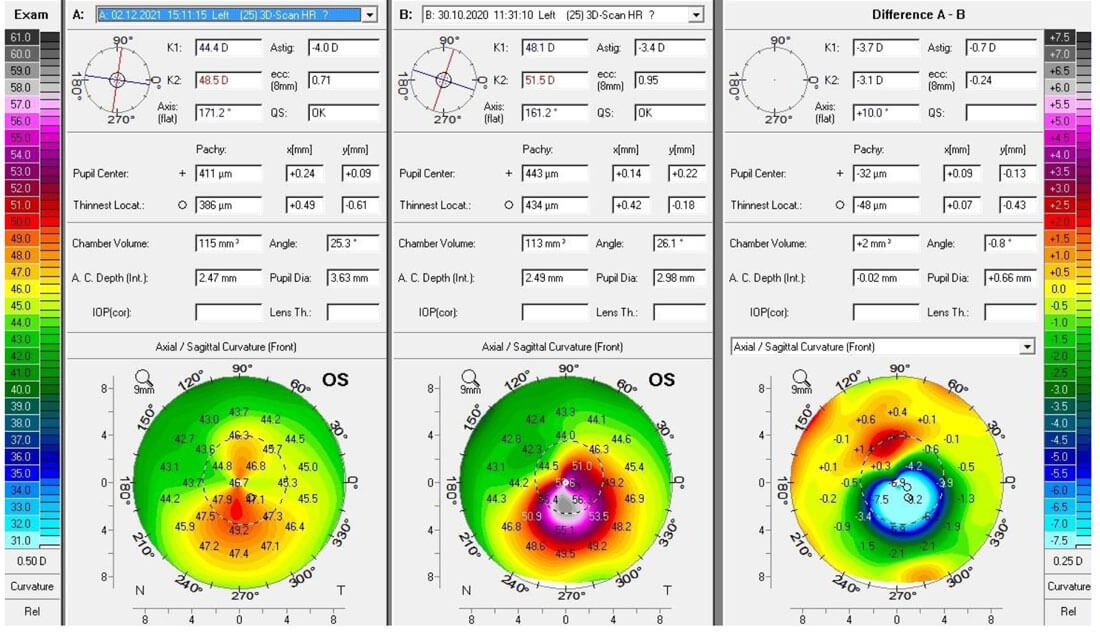
Difference map demonstrating improvement in corneal shape and irregularity.
Tomographic imaging such as optical cross-sections, ocular coherence tomography (OCT), and Scheimpflug imaging has been proved to be useful for the diagnosis of early keratoconus [10]. Such techniques allow imaging of the entire anterior segment, including the posterior cornea, and allow a three-dimensional structure reconstruction. This provides invaluable information, as after the 2015 Global Consensus of Keratoconus and Ectatic Disease, four multinational cornea societies concluded that an abnormal posterior cornea and / or an abnormal corneal thickness distribution is required to diagnose keratoconus [10]. Several authors have described the role of corneal epithelial thickness mapping with spectral-domain optical coherence tomography (SD-OCT) to screen for keratoconus, sometimes even in subclinical forms [7].
Patients with keratoconus reported significantly impaired vision-related quality of life (V-QoL), similar to that of patients with severe macular degeneration, as documented by the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) [8]. Given that keratoconus affects patients right from their early decades of life, the potential of quality of vision issues to impact quality of life, education, career decisions and capabilities is a significant socio-economic consideration, and so visual rehabilitation is an important aspect of the care provided to these patients. In practice, this is usually achieved with a combination of non-surgical and surgical approaches, dependent on the stage of the disease and the patient profile.
Non-surgical management of keratoconus
The corneal distortion in keratoconic eyes generally contributes to complex astigmatic and myopic corrections. Patients with early keratoconus may benefit from spectacle correction, however as the disease progresses, these can be limited in what benefit they provide both to visual acuity as well as the quality of vision, especially in low light conditions.
In early stages in the primary eyecare setting, patients may do well with soft contact lenses, however as the cornea distorts in cases of progression, more specialist methods of contact lens fitting may be employed. Practitioners have a variety of lens materials and types to best customise the fit and final visual outcome [11].
Types of contact lenses
Good lens selection and fitting helps most patients with keratoconus remain visually functional. Soft spherical or toric lenses may be useful in very early cases, however the mainstay for many decades has been rigid gas permeable (RGP) contact lenses. These have been shown to decrease higher order aberrations by around 60% [12]. Comfort can be a barrier to uptake in new patients, however, and in these instances, there are other lens options such as hybrid contact lenses (having a rigid centre and soft periphery), scleral lenses, and specialist soft lenses designed specifically for corneal distortions. Piggyback systems use two lenses, an RGP which is piggybacked onto a soft contact lens, and these show improved patient comfort and tolerance, including in post-corneal transplant patients [11]. Multi-curvature lenses such as Rose K lenses are also well suited to early cases, particularly with central nipple cones [11].
Given the large range of refractive aberrations as well as patients often being neurally adapted to their habitual blur, the fitting process proves complex for these patients [13]. It is important to balance a good fitting result for improved visual acuity and quality of vision as well as maximum comfort with reduced potential for impacting the health of the cornea and scarring. Availability of newer lens materials and designs, particularly with higher Dk values, has helped provide a larger range of options for practitioners and patients who are being managed both before and after surgical intervention [11].
Surgical management of keratoconus
Treatment protocols are generally based on a combination of assessing the severity of the keratoconus and the rate of progression in patients who are monitored regularly. While spectacles and contact lenses are often referenced as a treatment for the condition, they are only able to assist with refractive correction and improving visual acuity, especially in early to moderate cases [11]. They do not treat or cure the disease itself.
Photochemical corneal collagen cross-linking (CXL) has been accepted as the primary therapeutic option for keratoconus and other corneal ectasias over the past decades. In cases where the disease is progressing but not severe enough to require a corneal transplant, CXL aims to halt the progression of the disease by strengthening the cornea using ultraviolet A light and the chromophore riboflavin (vitamin B2). Refractive surgery combined with CXL, by way of photorefractive keratectomy (PRK) or topography-guided ablation with excimer lasers, is proving beneficial in improving postoperative visual outcomes. Corneal reprofiling in this way addresses the poor quality of vision issues in patients and assists with targeting improved best-corrected visual acuity (BCVA) or reduction in complexity of refraction, meaning that more contact lens options become available to these patients.
A 10-year outcomes study documented significant improvements in parameters such as uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), keratometry, spherocylindrical refraction, spherical equivalent (SE), spherical and cylindrical refraction, and corneal flattening with comparatively low rates and sometimes no complications observed [14]. Long-term outcomes are now also better understood, with one outcomes study of progressive keratoconus management with the Athens protocol (topography-guided partial refraction PRK combined with CXL) leading to increased confidence in safety and efficacy of these treatment protocols [15].
Other refractive procedures such as intrastromal corneal ring segments (ICRS) and phakic intraocular lens (PIOL) implantation are also options to enhance the visual outcome when combined with CXL. These may be more beneficial in patients with high refractive errors wishing to achieve more spectacle or contact lens independence. Studies suggest that intrastromal corneal ring segments such as INTACS are best indicated in patients with mild to moderate disease and who retain a clear cornea in the optical zone. Outcomes appear to be variable, with a range from two lines of loss of BCVA to eight lines of gain and 70 to 80% of patients showing improvement in BCVA and UCVA [15].
Long-term outcomes of PIOL implantation in keratoconus are promising. Hashemian et al. showed five-year outcomes for patients with the Visian ICMV4 PIOL (STAAR Surgical) indicating early stability of refraction achieved, remaining stable in the follow-up period [16]. Retrospective studies looking at patients fitted with iris-fixated PIOLs (Artisan / Artiflex (Ophtec®), Verisyse / Veriflex (AMO®)) also appear to suggest that patients with pellucid marginal degeneration (PMD)-like appearance ectasia might benefit the most from such procedures [17].
In severely advanced cases where there is also significant scarring in the visual axis, a corneal transplant or keratoplasty, either penetrating or deep anterior lamellar, may remain the only other surgical options. A study showed that despite satisfactory results on visual outcome measures following penetrating keratoplasty, the vision-related QoL using the National Eye Institute Visual Function Questionnaire (NEI-VFQ) remained impaired in these patients [18]. Long-term results of PK outcomes also show that although recovery of visual acuity can be good, the graft survival rate gradually decreased 20 years after PK [20]. Given the younger demographic of keratoconus graft recipients, these factors should influence patient counselling, and inevitably the need for early detection, monitoring and treatment, in order to leave other surgical options open.
Although there have been substantial advances in detecting and treating keratoconus in the past two decades, the ability to perform detailed corneal screening in primary care optometry practices remains a specialist application in most areas. Patient awareness and practitioner education tools would make reasonable routes to improving early keratoconus detection in the population.
TAKE HOME MESSAGE
The demographic profile of keratoconic patients has prompted the development of novel approaches to diagnosis, early detection, and treatment all contributing to addressing the socio-economic burden of the disease.
References
1. Keratoconus - Moorfields Eye Hospital.
https://www.moorfields.nhs.uk/
condition/keratoconus
2. Gordon-Shaag A, Millodot M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. Biomed Res Int 2015.
https://www.hindawi.com/journals/
bmri/2015/795738/
3. Mukhtar S, Ambati BK. Pediatric keratoconus: a review of the literature. Int Ophthalmol 2018. 2018;38(5):2257-66.
4. Keratoconus - Europe. American Academy of Ophthalmology.
https://www.aao.org/topic-detail/
keratoconus-europe
5. Kok YO, Ling Tan GF, Loon SC. Review: Keratoconus in Asia. Cornea 2012. 2012;31(5):581-93.
6. de Azevedo Magalhães O, Gonçalves MC, Gatinel D. The role of environment in the pathogenesis of keratoconus. Curr Opin Ophthalmol 2021. 2021;32(4):379-84.
7. Kundu G, Belin M, Shetty N, et al. ABCD: A new classification for keratoconus. Indian J Ophthalmol 2020;68(12):2831.
8. Wagner H, Barr J, Zadnik K. Collaborative longitudinal evaluation of keratoconus (CLEK) study: methods and findings to date. Cont Lens Anterior Eye 2007;30(4):223-32.
9. Duncan J, Gomes J. A new tomographic method of staging / classifying keratoconus: the ABCD grading system. International Journal of Keratoconus and Ectatic Corneal Diseases 2015;4(3):85-93.
10. Serrao S, Lombardo G, Calì C, Lombardo M. Role of corneal epithelial thickness mapping in the evaluation of keratoconus. Cont Lens Anterior Eye 2019;42(6):662-5.
11. Rathi V, Mandathara P, Dumpati S. Contact lens in keratoconus. Indian J Ophthalmol 2013;61(8):410.
12. Choi J, Wee W, Lee J, Kim M. Changes of ocular higher order aberration in on- and off-eye of rigid gas permeable contact lenses. Optom Vis Sci 2007;84(1):42-51.
13. Fischinger I, Wendelstein J, Tetz K, et al. Toric phakic IOLs in keratoconus - evaluation of preoperative parameters on the outcome of phakic anterior chamber lens implantation in patients with keratoconus. Graefes Arch Clin Exp Ophthalmol 2021;259(6):1643-9.
14. Kanellopoulos A. Ten-year outcomes of progressive keratoconus management with the Athens protocol (topography-guided partial-refraction PRK combined with CXL). J Refract Surg 2019;35(8):478-83.
15. Kanellopoulos A, Asimellis G. Keratoconus management: long-term stability of topography-guided normalization combined with high-fluence CXL stabilization (the Athens protocol). J Refract Surg 2014;30(2):88-93.
16. Hashemian S, Saiepoor N, Ghiasian L, et al. Long‐term outcomes of posterior chamber phakic intraocular lens implantation in keratoconus. Clin Exp Optom 2018;101(5):652-8.
17. Fukuoka S, Honda N, Ono K, et al. Extended long-term results of penetrating keratoplasty for keratoconus. Cornea 2010;29(5):528-30.
18. Yildiz E, Cohen E, Virdi A, et al. Quality of life in keratoconus patients after penetrating keratoplasty. Am J Ophthalmol 2010;149(3):416-22.
19. Volatier T, Figueiredo F, Connon C. Keratoconus at a molecular level: a review. Anat Rec 2019;303(6):1680-8.
20. Collier S. Is the corneal degradation in keratoconus caused by matrix-metalloproteinases? Clin Exp Ophthalmol 2001;29(6):340-4.
[All links last accessed January 2023].
Declaration of competing interests: None declared.
Images: Courtesy of AccuVision: The Eye Clinic.
COMMENTS ARE WELCOME