The author provides an update on late breaking clinical trial results in neovascular age-related macular degeneration (nAMD) and presentations on diabetes management from the American Academy of Ophthalmology Retina Subspecialty Day, held during the Academy’s annual meeting in Chicago, October 2018.
Late breaking developments and clinical trial results in nAMD
Brolucizumab in nAMD: HAWK and HARRIER 96-week results
In the HAWK and HARRIER phase 3 nAMD studies, the primary endpoint of non-inferiority of brolucizumab 6mg (Novartis) to aflibercept (Eylea®, Regeneron, Bayer) in mean change in best corrected visual acuity (BCVA) from baseline was met at week 48 [1]. The study design of both trials involved a matched loading phase up to week 16, followed by a maintenance phase of fixed brolucizumab dosing every 12 weeks with an option to adjust to fixed eight-weekly dosing based on masked disease activity assessment at defined visits (q12w/q8w) or aflibercept given every eight weeks (q8w) through 96 weeks.
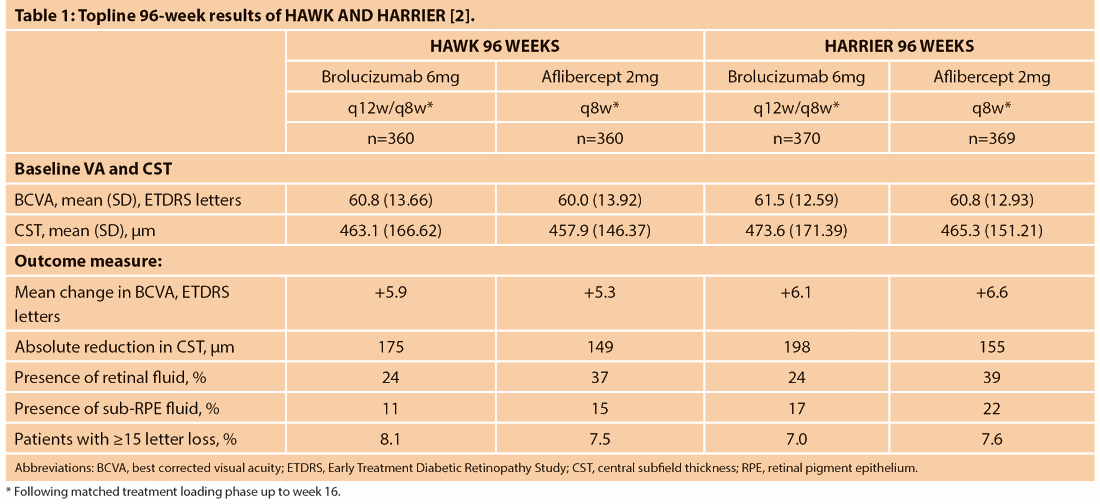
The BCVA achieved by brolucizumab at week 48, the primary endpoint, was maintained at week 96 [2]. Table 1 summarises topline results at 96 weeks [2]. Successful completion of q12w brolucizumab dosing at week 48 was highly predictive of continuing on that same quarterly treatment interval until week 96. Of the patients on brolucizumab 6mg who successfully completed year one on a q12w dosing interval (i.e., 56% in HAWK and 51% in HARRIER), 82% in HAWK and 75% in HARRIER were maintained on a q12w dosing interval in year two (overall, ~46% in HAWK and 38% in HARRIER). The overall safety profile of brolucizumab was comparable to aflibercept.
Use of aflibercept treat-and-extend in ALTAIR: 96-week outcomes
The updated EU label for aflibercept permits treat-and-extend dosing beyond every eight weeks in the first year of treatment for nAMD, with flexibility to extend or adjust by four- or two-week increments, based on evidence of efficacy from the Japanese ALTAIR clinical trial [3].
The ALTAIR study assessed the efficacy and safety of aflibercept therapy using two different treat-and-extend regimens beyond every-eight-weeks (with either four- or two-week adjustment increments, minimum treatment interval of eight weeks and a maximum extension of 16 weeks) for treatment naive nAMD patients through 96 weeks. Mean BCVA gains from baseline (~55 letters) were 8.4 to 9.0 letters at week 52 and 6.1 to 7.6 letters at week 96 for the four-week and two-week adjustment groups, respectively [4-6]. There was a mean of 10.4 injections given overall, with a mean of 3.6 to 3.7 injections in the second year. At the week 52 study visit, 57% of study patients had a next intended injection interval of ≥12 weeks based on the next scheduled treatment, and almost 60% of the overall study population had a last injection interval ≥12 weeks at final study visit at week 96 [Figure 1]. Results demonstrate that extended dosing with aflibercept after an initial loading phase is effective in improving and maintaining vision through 96 weeks, with a reduced treatment burden in the second year and a majority extended to quarterly or longer dosing intervals.
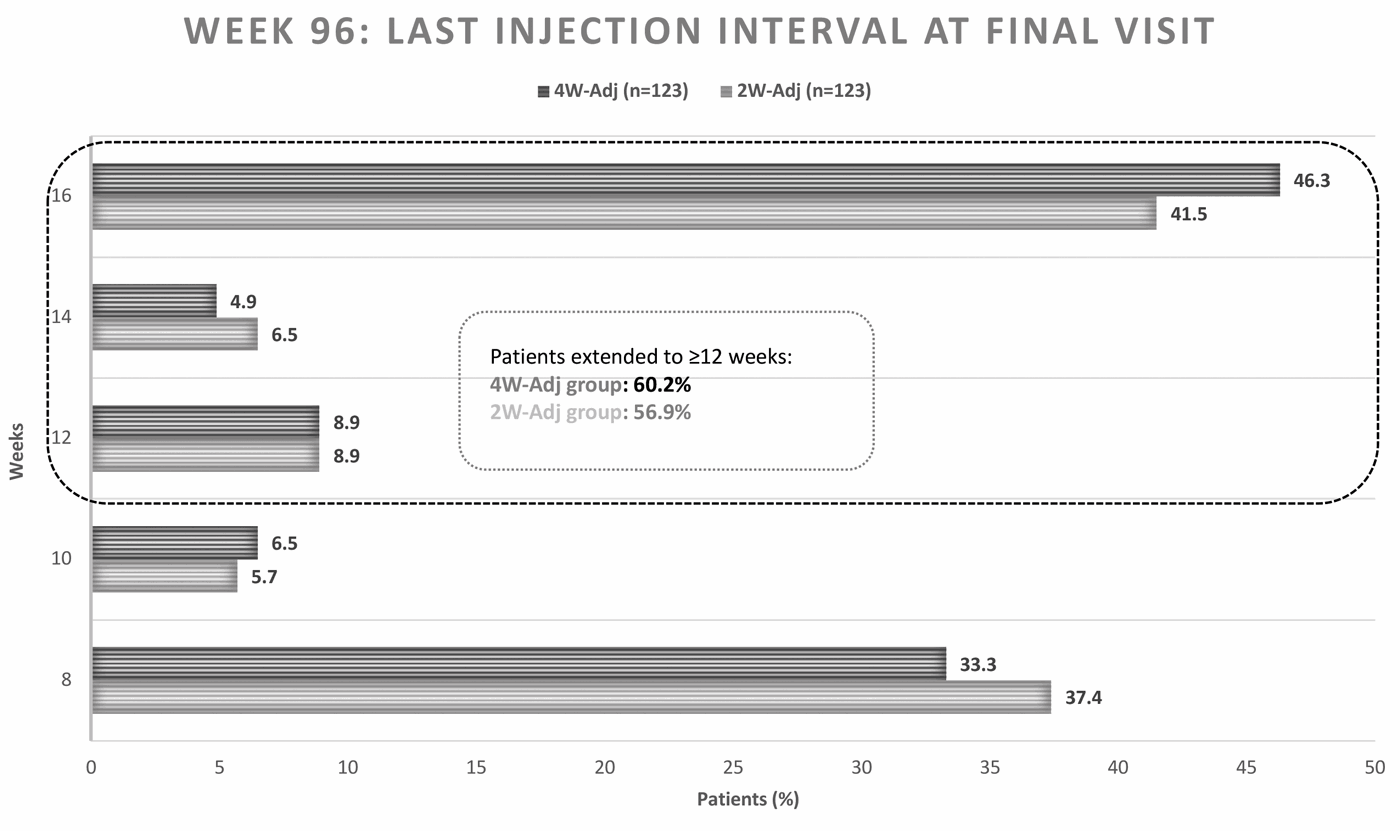
Figure 1: ALTAIR 96-week results showing last injection interval at final study visit week 96 [5,6].
Safety and efficacy of abicipar in patients with nAMD
SEQUOIA and CEDAR are two randomised, double-masked, parallel-group phase 3 clinical trials designed to evaluate the safety and efficacy of intravitreal abicipar pegol (Allergan) compared with ranibizumab (Lucentis®, Novartis) in treatment-naive patients with nAMD. Both abicipar q8w and q12w regimens (after three loading doses at weeks one, four and 12) met the prespecified criteria for non-inferiority to monthly ranibizumab for stable vision (loss of <15 letters from baseline) at week 52 in both trials, with more than 91% of abicipar patients achieving stable vision [7]. The q8w and q12w regimens in SEQUOIA and the q8w regimen in CEDAR met the prespecified criteria for non-inferiority to ranibizumab for the key secondary efficacy endpoint mean BCVA change from baseline at week 52. Reductions from baseline in central retinal thickness (CRT) at week 52 were similar between abicipar treatment arms (six to eight injections) and the ranibizumab treatment arm (13 injections) in both SEQUOIA and CEDAR. BCVA and CRT improvements after the initial loading phase were maintained at week 52.
Abicipar-treated patients had a higher risk of developing intraocular inflammation than ranibizumab-treated patients (overall, 15.3%-15.4% for abicipar-treated patients compared with 0.3% for the ranibizumab arm). Most abicipar-treated patients with intraocular inflammation had events of mild to moderate severity and were treated with topical corticosteroid drops. Allergan expects results from the MAPLE trial using its further optimised formulation in first half 2019.
Simultaneous inhibition of Ang-2 and VEGF with faricimab in nAMD: STAIRWAY phase 2 results
Combined inhibition of angiopoietin-2 (Ang-2) and vascular endothelial growth factor (VEGF) can reduce vascular destabilisation and leakage, and shows potential for increased durability in nAMD. Faricimab (Roche) is a bispecific antibody designed specifically for intravitreal use to simultaneously bind to and neutralise both Ang-2 and VEGF-A with high potency and specificity.
The phase 2 STAIRWAY clinical trial evaluated 6mg faricimab dosed either every 16 weeks or every 12 weeks compared to monthly ranibizumab in patients with nAMD. Results show 65% of faricimab-treated patients had no disease activity 12 weeks after the last loading dose, while gains in BCVA from baseline were maintained through week 52, with both faricimab dosing regimens comparable to monthly ranibizumab (+11.42, +10.08 and +9.59 letters from mean VA at baseline of 60.4, 57.8 and 55.3 letters for faricimab q16w, faricimab q12w and monthly ranibizumab groups, respectively). Anatomic improvements were comparable between faricimab treatment regimens and monthly ranibizumab [8]. Treatment with faricimab was well tolerated with no new safety signals identified.
Port Delivery System implant with ranibizumab
Study results from the ongoing phase 2 LADDER study of the investigational Port Delivery System with ranibizumab (PDS, Roche) for nAMD were presented [9]. In the PDS ranibizumab 100mg/mL treatment arm (n=59), 80% of patients (previously responsive to anti-VEGF therapy) went ≥6 months until the first refill (primary analysis performed when last patient completed the month nine visit). Median time to first required refill was 15.0 months, and BCVA and anatomic outcomes between PDS with ranibizumab 100mg/mL and monthly intravitreal ranibizumab were comparable, with improved or maintained vision over nine months (from mean BCVA at baseline of ~70 letters, mean change of +4.3 letters in the PDS 100mg/mL arm and +3.3 letters in the monthly intravitreal ranibizumab arm at month nine). Subjects in the PDS 100mg/mL group (n=59) required a mean 2.4 ranibizumab treatments over 16.4 months compared with 16.8 injections in the monthly ranibizumab arm (n=41). Postoperative vitreous haemorrhage rate in the PDS arm was significantly reduced after a modified surgical technique was implemented in the course of the trial. The phase 3 Archway clinical trial, comparing fixed 24-week PDS refills vs. monthly ranibizumab injections, began enrolling in September 2018.
Diabetes: lessons learned, new treatment paradigms for diabetic macular oedema and diabetic retinopathy
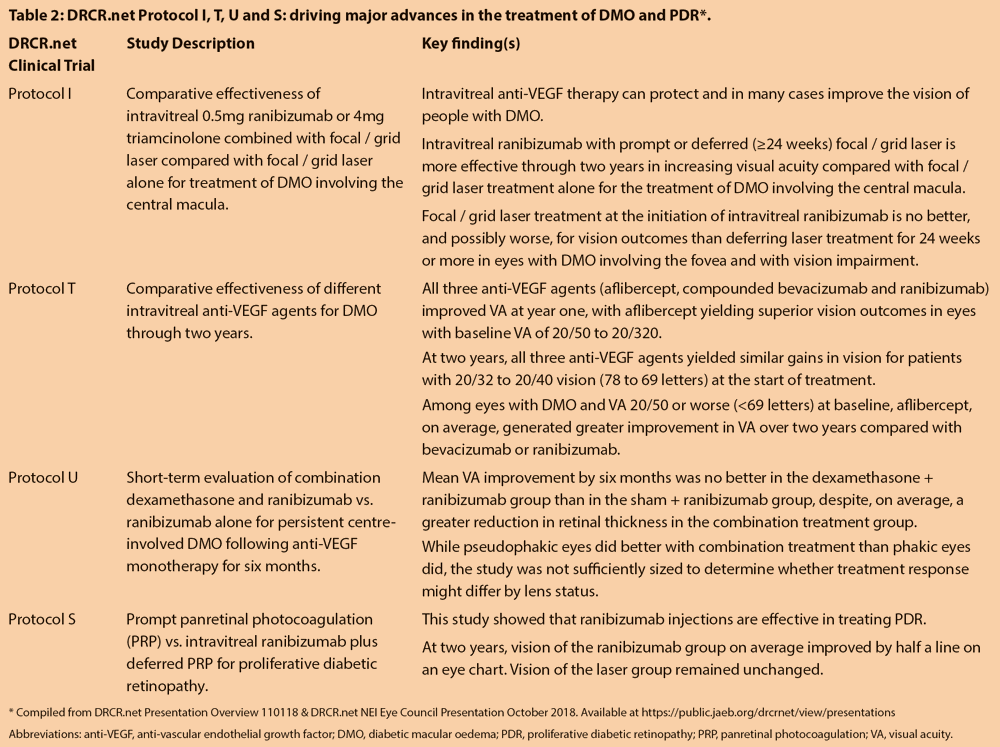
Anti-VEGF therapy is now the mainstay of treatment of diabetic macular oedema (DMO) and regarded as a viable alternative to panretinal photocoagulation (PRP) for proliferative diabetic retinopathy (PDR), explained Dr John A Wells III, West Columbia, SC, USA. In a review of landmark Diabetic Retinopathy Clinical Research Network (DRCR.net) studies that have changed treatment practice for diabetic-related retinal disease, he underscored core lessons learned from DRCR.net Protocol trials I, T, U and S (Table 2) [10-15]:
- Treatment of centre-involved DMO with some vision loss should be initiated with anti-VEGF therapy and focal / grid laser deferred for at least six months (Protocol I).
- The treatment burden following initial intensive treatment in DMO declines over time (Protocols I and T).
- Aflibercept is more effective than ranibizumab or compounded bevacizumab in centre-involving DMO eyes with starting VA 20/50 or worse (Protocol T).
- Aflibercept, bevacizumab or ranibizumab can be given in eyes with baseline VA 20/32 to 20/40, recognising, however, that bevacizumab reduces optical coherence tomography (OCT) oedema less effectively and is more likely to result in persistent DMO, which may also lead to inferior vision outcomes in eyes with OCT thickness greater than 400 microns (Protocol T).
- Adding steroids for persistent DMO following anti-VEGF therapy is not beneficial. In eyes with persistent DMO after at least six months of anti-VEGF therapy, continuing anti-VEGF monotherapy can result in continued improvement (Protocol U). In the Protocol U study, mean (SD) VA change from randomisation to week 24 was +2.7 (9.8) letters in the dexamethasone (Ozurdex®, Allergan) + ranibizumab combination group and +3.0 (7.1) letters in the ranibizumab group, adjusted treatment group difference (combination minus ranibizumab) of -0.5 letters (95% CI, -3.6 to 2.5; P=0.73).
- In eyes with PDR and coexisting DMO, ranibizumab treatment alone should be considered over PRP plus ranibizumab, though the benefit declines from two to five years of treatment (Protocol S).
- While the benefits of ranibizumab to vision and visual fields declined over time, secondary complications of PDR, such as development of DMO, tractional retinal detachment and the need for vitrectomy were less in the ranibizumab group than the PRP group over five years (Protocol S). Rates of vitreous haemorrhage were equal. These benefits need to be weighed against the increased treatment burden and risk of noncompliance associated with ranibizumab monotherapy for PDR.
Association between early response and long-term outcomes with anti-VEGF therapy for DMO
An analysis of outcomes from Protocol T showed that an early suboptimal VA response (<5-letter gain from baseline at 12 weeks) to anti-VEGF treatment did not preclude meaningful vision improvement (≥15 letter gain) at two years without switching therapies, with the same proportion of eyes achieving 20/40 vision or better at two years whether gaining <5 letters or gaining ≥10 letters after three initial injections [16].
With respect to initial anatomic response to treatment, an independent post hoc analysis of Protocol I data found that a strong anatomical response (≥20% reduction at 12 weeks) to ranibizumab was largely maintained during long-term treatment and was associated with greater long-term improvement in VA [17]. However, after controlling for potential confounders including baseline vision and CRT and prior DMO treatment, the association with long-term visual response lost statistical significance.
Changes in DR severity with anti-VEGF therapy for DMO
Anti-VEGF therapy can improve retinopathy severity in a substantial proportion of DMO patients and appears to significantly reduce the risk of retinopathy worsening. Dr Susan Bressler, Baltimore, MD, USA, discussed DRCR.net Protocol I five-year rates of retinopathy improvement or worsening among eyes assigned to ranibizumab in the context of progressive reduction in exposure to ranibizumab therapy [18]. Individuals managed with anti-VEGF therapy for DMO may have simultaneous favourable changes in diabetic retinopathy (DR) severity through five-year follow-up. In an exploratory analysis, improvement and worsening rates did not appear to be altered by the marked reduction in exposure to ranibizumab during the later years of follow-up. However, individual eyes may not maintain improvement, and worsening does occur, highlighting the need for continued surveillance of DR level. The cumulative probability of worsening through five years was 18% among non-proliferative DR eyes and 31% among PDR eyes [18].
Dr Quan Dong Nguyen, MD, Palo Alto, CA, USA, presented findings of a meta-analysis of regression of DR from four similar randomised clinical trials involving ranibizumab in eyes with DR and DMO [19]. Treatment of earlier stages of DR with intravitreal anti-VEGF inhibitors may be considered to prevent or decrease the risk of developing vision-threatening PDR, argued Dr Nguyen. In DRCR.net Protocol T, low rates of DR worsening were observed with all three anti-VEGF treatments. For those with PDR at baseline, significantly higher rates of improvement of DR severity over two years were observed with aflibercept treatment compared with bevacizumab or ranibizumab (improvement rate at one year of 75.9%, 31.4% and 55.2%, respectively, rates and treatment group differences maintained at two years) [20].
What happens to patients after they leave the DRCR network studies?
Dr David J Browning, Charlotte, NC, USA, discussed the gap between clinical study and real world outcomes of intravitreal anti-VEGF therapy for DMO. Data from randomised controlled clinical trials demonstrate the efficacy of anti-VEGF drugs at improving vision in subjects with DMO, with mean VA gains of two lines or more from baseline at one year. However, real world regimens appear to work less well than clinical trial protocols, with fewer visits and injections correlating with less improvement in VA [21]. Holekamp et al. reported real world cohort outcomes in DMO showing a mean corrected VA change of +4.7 letters (mean 56.9 letters at baseline) in the first 12 months, with a mean number of intravitreal injections of 3.1 (range, 1-12) [21]. This gap, suggested Dr Browning, may be closed by introducing case managers, expanding injection delivery capacity, performance audits, pragmatic protocols and consideration of different models of care, e.g. fixed-schedule injections and less clinical monitoring.
Diabetic patients underutilise eye care services and are prone to significant lapses in follow-up, due to unanticipated health issues, financial hardship or noncompliance, explained Dr Mark W Johnson, MD, Ann Arbor, MI, USA. For example, of 10 million diabetic patients evaluated in the emergency department annually in the United States, nearly six million are hospitalised [22]. Over 40% of patients with DMO have at least one loss to follow-up of >100 days [23], while Obeid et al. reported loss to follow-up after treatment for PDR occurred in 25.4% of patients over four years [24]. Eyes with PDR treated solely with anti-VEGF injections have worse anatomic and functional outcomes after losses to follow-up compared with eyes that receive PRP [25]. Practitioners were encouraged to carefully consider potentially severe consequences of interruptions in anti-VEGF therapy, and likely compliance with follow-up and treatment regimen, when making initial treatment decisions.
References
1. Dugel P, et al. HAWK & HARRIER: 48-week results of 2 multi-centered, randomized, double-masked trials of brolucizumab versus aflibercept for neovascular AMD. Presentation at the American Academy of Ophthalmology 2017 Annual Meeting on 10 November 2017, New Orleans, USA.
2. Dugel P, et al. Phase 3, randomized, double-masked, multi-center trials of brolucizumab versus aflibercept for neovascular AMD: 96-week results from the HAWK and HARRIER studies. Presentation at the American Academy of Ophthalmology 2018 Annual Meeting on 27 October 2018, Chicago, USA.
3. Eylea® (aflibercept solution for injection) [summary of product characteristics]. Leverkusen, Germany: Bayer AG; 2018.
https://www.medicines.org.uk/
emc/medicine/27224
Last accessed December 2018.
4. Ohji M, et al. Two different treat and extend dosing regimens of intravitreal aflibercept for wAMD in Japanese patients: 52 weeks results of the ALTAIR study. Presentation at the 17th European Society of Retina Specialists (EURETINA) Congress on 7 September 2017; Barcelona, Spain.
5. Ohji M, et al. Two different treat-and-extend dosing regimens of intravitreal aflibercept in Japanese patients with wet age-related macular degeneration: 96-week results of the ALTAIR study. Presentation at the 18th European Society of Retina Specialists (EURETINA) Congress on 21 September 2018; Vienna, Austria.
6. Ohji M, et al. Use of intravitreal aflibercept treat-and-extend dosing for wet age-related macular degeneration: 96-week ALTAIR results. Presentation at the American Academy of Ophthalmology 2018 Annual Meeting on 27 October 2018, Chicago, USA.
7. Khurana R. Safety and efficacy of abicipar in patients with neovascular age-related macular degeneration. Presentation at the American Academy of Ophthalmology 2018 Annual Meeting on 26 October 2018, Chicago, USA.
8. Khanani AM. Simultaneous inhibition of VEGF and Ang-2 with faricimab in neovascular AMD: STAIRWAY phase 2 results. Presentation at the American Academy of Ophthalmology 2018 Annual Meeting on 26 October 2018, Chicago, USA.
9. Regillo CD. Port Delivery Phase 2 LADDER AMD study results. Presentation at the American Academy of Ophthalmology 2018 Annual Meeting on 26 October 2018, Chicago, USA.
10. Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular oedema. Ophthalmology 2010;117(6):1064-77.
11. Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015;372(13):1193-203.
12. Wells JA, Glassman AR, Ayala AR, et al; Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 2016;123(6):1351-9.
13. Maturi RK, Glassman AR, Liu D, et al; Diabetic Retinopathy Clinical Research Network. Effect of adding dexamethasone to continued ranibizumab treatment in patients with persistent diabetic macular edema: a DRCR Network Phase 2 randomized clinical trial. JAMA Ophthalmol 2018;136(1):29-38.
14. Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, Jampol LM, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol 2015;314(20):2137-46.
15. Gross JG, Glassman AR, Liu D, et al; Diabetic Retinopathy Clinical Research Network. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol 2018;136(10):1138-48.
16. Bressler NM, Beaulieu WT, Maguire MG, et al; Diabetic Retinopathy Clinical Research Network. Early response to anti-vascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in Protocol T. Am J Ophthalmol 2018;195:93-100.
17. Dugel PU, Campbell JH, Kiss S, et al. Association between early anatomic response to anti-vascular endothelial growth factor therapy and long-term outcome in diabetic macular edema: an independent analysis of Protocol I study data. Retina 2019;39(1):88-97.
18. Bressler SB, Odia I, Glassman AR, et al. Changes in diabetic retinopathy severity when treating diabetic macular edema with ranibizumab: DRCR.net Protocol I 5-year report. Retina 2018;38(10):1896‑1904.
19. Nguyen QD. Regression of diabetic retinopathy with anti-VEGF treatment: meta-analysis of 4 pivotal clinical trials. Presentation at the American Academy of Ophthalmology 2018 Annual Meeting on 27 October 2018, Chicago, USA.
20. Bressler SB, Liu D, Glassman AR, et al; Diabetic Retinopathy Clinical Research Network. Change in diabetic retinopathy through 2 years: secondary analysis of a randomized clinical trial comparing aflibercept, bevacizumab, and ranibizumab. JAMA Ophthalmol 2017;135(6):558-68.
21. Holekamp NM, Campbell J, Almony A, et al. Vision outcomes following anti-vascular endothelial growth factor treatment of diabetic macular edema in clinical practice. Am J Ophthalmol 2018;191:83‑91.
22. Korbel L, Spencer JD. Diabetes mellitus and infection: an evaluation of hospital utilization and management costs in the United States. J Diabetes Complications 2015;29(2):192-5.
23. Weiss M, Sim DA, Herold T, et al. Compliance and adherence of patients with diabetic macular edema to intravitreal anti-vascular endothelial growth factor therapy in daily practice. Retina 2018;38(12):2293-300.
24. Obeid A, Gao X, Ali FS, et al. Loss to follow-up in patients with proliferative diabetic retinopathy after panretinal photocoagulation or intravitreal anti-VEGF injections. Ophthalmology 2018;125(9):1386‑92.
25. Obeid A, Su D, Patel SN, et al. Outcomes of eyes lost to follow-up with proliferative diabetic retinopathy that received panretinal photocoagulation versus intravitreal anti-vascular endothelial growth factor. Ophthalmology 2018 [Epub ahead of print].
COMMENTS ARE WELCOME