Glaucoma is a challenging condition to treat because the exact pathophysiology remains unclear and the only readily modifiable factor is the intraocular pressure (IOP). Additionally, most glaucoma patients are completely asymptomatic, yet are often subjected to lifelong medical therapy. The problems of adherence, persistence and side-effects of glaucoma drops are well recognised clinically and well published academically [1].
Conventional surgical treatment for glaucoma has been proven to be highly effective over the long- term [2], but results vary significantly and the complication rate remains a concern for clinicians and patients. It is therefore not surprising to find a growing amount of interest in recent years to seek alternative surgical treatments that are effective in lowering IOP and reducing medication burden, but which are accompanied by a better safety profile.
Minimally invasive glaucoma surgery (MIGS) has been the ‘hot topic’ in glaucoma over the last decade. Procedures classified as MIGS are quick, performed through a micro-corneal incision without disruption of the sclera and conjunctiva (ab-internally), have fast surgical recovery and no postoperative manipulation. Trabectome (Neomedix Corporation, Tustin, California, USA) was first developed to remove trabecular meshwork (TM) over three to four clock hours exposing the underlying Schlemm’s canal and collector channels. The iStent (Glaukos Corporation, Laguna Hills, CA, USA) was designed to be implanted at the angle to bypass trabecular meshwork, allowing a better point of access to the canal.
This concept was then further expanded by the Hydrus Microstent (Ivantis Inc, Irvine, CA), which not only bypasses the trabecular meshwork, but also scaffolds the canal over three clock hours. Clinical results of Trabectome and iStent have been well published and the adoption of these procedures has been worldwide. Hydrus data is slowly emerging but it is not yet a commercial product. Overall these procedures offer similar efficacy with significant IOP reduction to the mid to high teens, even with a reduction of one to two medications [3,4]. They are also often combined with cataract surgery for the extra IOP lowering effect phacoemulsification offers [5].
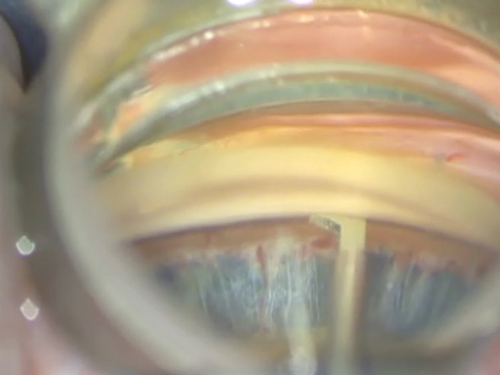
Figure 1: Xen45 gel implant is 6mm long with an internal lumen of
45um and designed to create an external drainage bleb.
While the previously mentioned procedures were designed to enhance physiological aqueous outflow, other forms of MIGS are gaining popularity by exploring the traditional subconjunctival drainage concept. The XEN45 gel implant (Allergan PLC, Dublin, Ireland) and InnFocus Microshunt (Santen Pharmaceutical Co., Osaka, Japan) are drainage implants that form a fistula between the anterior chamber and subconjunctival / sub-Tenon’s space (Figure 1). While the XEN45 is implanted ab-internally, the InnFocus is implanted ab-externally after conjunctival dissection. The more invasive implantation technique, especially the latter, combined with the use of antifibrotics and the need for postoperative bleb management, has taken these devices slightly out of the MIGS arena, albeit with a favourably low complication rate. The efficacy of both of these implants appears to be superior to iStent, Hydrus and Trabectome and may approach the IOP lowering effect of trabeculectomy [6,7], with potentially less attendant medication and follow-up burden, but data remains limited.
While the glaucoma community is still trying to digest the above information and decide on which technologies to adopt, largely on the basis of pilot data, another wave of new MIGS is already emerging onto the market, fully commercially available but often with even more limited data and a lack of published randomised control trials (RCT) results. Here we would like to discuss some of these options.
Figure 2: Kahook dual blade is designed to remove trabecular
meshwork ab-internally under gonioscopic guidance.
Trabecular meshwork bypass / removal
It has been well demonstrated that increased trabecular meshwork resistance is a contributing factor to increased IOP [8]. Trabecular meshwork removal, by way of goniotomy or Trabectome has demonstrated significant IOP reduction [9]. The Kahook Dual Blade (New World Medical, Rancho Cucamonga, CA) has been designed to simulate trabectome surgery by ab-internally removing TM over the same three to four clock hours (Figure 2). It is a single use device that makes parallel incisions in the TM and inner wall of Schlemm’s canal and is far cheaper and simpler than the Trabectome. However, it does not provide any anterior chamber stability and does not provide suction. A pre-clinical study demonstrated that the KDB blade removed TM more thoroughly and with less collateral damage than a simple MVR blade or Trabectome [10].
There have been no published results in the literature of ab-interno trabeculotomy performed with the KDB blade as yet. In-vitro whole eye perfusion studies suggest promising pressure lowering [10], albeit without assessing the in-vivo risk of intraoperative hyphaema.
An alternative to this concept is a 360 degrees ab-interno trabeculotomy termed gonioscopy-assisted transluminal trabeculotomy (GATT). During GATT surgery a small incision is made at the TM ab-internally under gonioscopic view. An illuminated micro-catheter or a suture [11] is then passed into Schlemm’s canal circumferentially until it emerges back through the original entry. The micro-catheter or suture is than pulled into the centre of the anterior chamber, tearing the TM and producing a 360 degree trabeculotomy. A retrospective case series of 57 patients with primary open angle glaucoma (POAG) showed a 11mmHg (40%) reduction in IOP with 1.1 fewer glaucoma medications at 12 months [12]. In a separate secondary glaucoma cohort, the IOP decreased by 19.9mmHg (56.8%) with an average of 1.9 fewer medications at 12 months. The main complication was hyphaema, which resolved by month one in 96% of patients.
“Although some of the newest additions appear to be modifications to some tried and tested procedures, their efficacy is yet to be proven and a lot more work is required to guide us through this maze.”
The technique has also been used successfully in more challenging angles in primary congenital glaucoma [13]. Unlike the KDB knife or the trabectome there is no removal of tissue. The cleavage plane in the trabecular meshwork is anterior creating a posterior leaflet of the trabecular meshwork that often becomes tethered to the peripheral iris [14]. Whether scarring occurs in the long term, compromising the success of the procedure, is yet to be observed.
Ab-interno canaloplasty
As well as increased resistance across the TM, collapse of Sclemm’s canal and blockage of collector channels by herniation of TM has also been demonstrated in POAG patients [15]. Procedures to dilate and restore Schlemm’s canal architecture and function have been performed for more than 30 years. Viscocanalostomy, first described by Stegmann in 1985, involves an external dissection of two scleral flaps to expose Schlemm’s canal, peeling off juxta-canalicular TM to allow a steady egress of aqueous, followed by viscodilation of the adjacent (but not 360 degree) Schlemm’s canal. The inner scleral flap is excised creating a scleral lake while the outer scleral flap is sutured tightly to avoid bleb formation. An enhancement of this technique, termed canaloplasty, was described by Lewis in 2007, where instead of viscodilating part of the canal, a suture is passed 360 degrees around the canal and tied at a specific tension to keep the canal stretched and dilated [16].
The exact mechanism of IOP reduction remains unclear, but it is thought to be a combination of canal dilation and increased corneal-scleral outflow. Subconjunctival outflow in the form of conjunctival cystic changes has also been demonstrated. Overall the results demonstrated significant IOP lowering to around 15mmHg at three years [17]. An IOP of <18 mmHg was achieved in 36% of patients without medications and in 77.5% with medications, although complete success without medications was significantly higher at 70.4% when the procedure was combined with cataract surgery. Although the efficacy of canaloplasty is inferior to trabeculectomy, there is a better safety profile and easier postoperative journey [17,18], and as ever the individual requirements of the patient should drive how the risks and benefits of each procedure are balanced.
Attempts have been made to try to mimic the results of canal-based surgery in a minimally invasive fashion. The Hydrus Microstent, as previous mentioned, claimed not only to bypass TM resistance, but also to dilate and scaffold the canal. Early histological evidence supported this theory [18] and the most recent study by Gandolfi et al. demonstrated very similar efficacy between the Hydrus Microstent and canaloplasty [19], although it was a retrospective comparison rather than a RCT. Ab-interno canaloplasty (ABiC) is a procedure where, similarly to the GATT procedure, an illuminated micro-catheter (iTrack, Ellex, Adelaide, Australia) is passed circumferentially into Schlemm’s canal ab-internally under gonioscopic guidance. However, instead of tearing out through the TM, the micro-catheter is retracted backwards while viscoelastic is injected into the canal. The aim is to viscodilate the canal and unblock the collector channels by potentially releasing herniated TM (Figure 3).

Figure 3: Ab-interno canaloplasty – micro-catheter is being withdrawn
while viscoelastic is injected into the Schlemm’s canal.
The illuminated tip is visible at 10 o’clock position at the limbus.
Whether the efficacy of ABiC approaches that of viscocanalostomy remains to be proven, as there are no peer-reviewed published clinical outcomes of this procedure so far. A white paper issued by the manufacturers based on the surgical outcomes of surgeons in two centres on 228 patients, claimed a reduction in IOP from 19mmHg on two medications to 13.3mmHg on one medication at 12 months, with combined cataract surgery not significantly affecting the outcome [20].
Supra-choroidal drainage
There have been attempts in the past to divert aqueous into the suprachoroidal space, the SOLX Gold Shunt (SOLX Inc, MA, USA) being the most well known of these previously being the most well known of these. Its efficacy has not been well reproduced and device blockage by ingrowing fibrotic tissue has been demonstrated [21]. Two other devices with a similar design, also designed to be implanted ab-externo, the STARflo Glaucoma Implant (iSTAR Medical SA) and the Aquashunt (previously OPKO Health Miami, USA) have been developed, although the Aquashunt no longer has any active clinical trials.
In recent years there is a resurgence of interest in this space in the form of two micro-stents: CyPass (Transcend Medical, Inc., Menlo Park, CA) and iStent supra (Glaukos Corporation, Laguna Hills, CA, USA). Both devices share similar design features and are both implanted ab-internally in an almost identical manner. The recently published COMPASS Study compared cataract surgery with concomitant CyPass insertion with cataract surgery alone [22]. Mean IOP reduction was 7.4mmHg for the microstent group (from 24.5mmHg) versus a 5.4mmHg in controls (P<0.001). Importantly, 85% of microstent subjects versus 59% of controls were medication free. There were no vision threatening adverse events.
The procedure has been developed further by combining stent insertion with injection of ophthalmic viscosurgical device to help maintain the suprachoroidal aqueous lake (termed Viscopass). A clinical trial comparing this technique with Cypass insertion alone is currently recruiting. There are no published results for the iStent Supra so far.
In-flow procedure
Although raised IOP in glaucoma is not thought to be an inflow issue, treatment to the ciliary body processes to reduce aqueous production, both pharmacologically and surgically, has served an invaluable role. Conventional cyclodiode laser has a proven track record in IOP reduction, especially in complicated and refractory glaucoma [23]. More recently, the adoption of endoscopic cyclophotocoagulation, especially combined with phacoemulsification, has shown significant IOP and medication reduction of a similar magnitude to current MIGS devices [24]. Unfortunately, cyclodestruction procedures are often associated with a higher rate of complications such as persistent inflammation, hyopotony and cystoid macular oedema.

Figure 4: The latest version of the HIFU probe during treatment.
Efforts have therefore been made to explore safer methods of ciliary body treatment. High-intensity focused ultrasound (HIFU) is a technology used successfully in the treatment of uterine fibroid and prostate disease. It has been adopted for glaucoma use by EyeTechCare, France using a device consisting of a ring-shaped probe embedded with six piezo-electric crystals that sits directly on the eye (Figure 4). Focused ultrasound is used to target the ciliary body and histological studies have demonstrated ciliary epithelium destruction and potentially an increase in ciliary outflow [25]. HIFU was originally licensed and marketed to treat refractory glaucoma.
There has been a series of prospective trials under the acronym EyeMust sponsored by the manufacturer. The pilot study in patients with refractory glaucoma, with at least one glaucoma procedure previously, showed a substantial reduction in IOP from 37.9mmHg at baseline to 24.7mmHg at up to one year [26]. The most recent study was conducted in POAG patients who had not had previous surgery. This showed a reduction in IOP from 28.2mmHg to 19.6mmHg at 12 months [27]. However, as with the previous studies, the final IOP was achieved on 3.1 medications, (compared to 3.6 at baseline) which is greater than many of the other procedures described here and is in contrast to the majority of published results on photocyclocoagulation. Complications included persistent anterior chamber flare at one month in 33% of patients, corneal ulceration in 17% of patients and corneal thinning in one patient. Studies are ongoing and its efficacy and safety, especially in pre-surgical eyes, remains to be proven. A further drawback of this procedure is that anterior segment measurements are required preoperatively to accurately locate the cilliary body so that a probe of the correct size can be used.
An alternative cyclodiode modality, termed Micropulse cyclophotocoagulation (Iridex, USA), is currently available commercially. The technology allows a continuous-wave laser beam to be divided into a train of shorter, repetitive, low energy pulses separated by a brief rest period. This allows the tissue to cool and is intended to reduce thermal tissue damage. A study from the National University Hospital, Singapore on 38 patients demonstrated a reduction in IOP from 40.1mmHg to 24.6mmHg at final follow-up [28]. IOP lowering medications were reduced from 2.1 before micropulse to 1.3 at final follow-up. There were no serious complications, although larger series would be required to demonstrate improved safety over trans-scleral diode. Similarly to HIFU, its efficacy in mild to moderate glaucoma is yet to be proven. More importantly, the safety profile of a ciliary body based treatment requires careful evaluation. Although these treatments are not truly marketed as MIGS, low complication rate and high patient satisfaction are important criteria that all new procedures should fulfill.
Conclusion
In order for medical treatment to advance there has to be a close collaboration between clinicians and industry. Those clinicians involved in such innovations are ultimately grateful for the vast amount of interest and investment into this field, yet are fully aware of the effect of any potential commercial pressure. It is our view that all clinicians interested in the early adoption of these new procedures should have an open but critical approach, proceed cautiously and have a robust self-auditing and reporting process in place. While we eagerly await longer-term data from our ‘first wave’ of MIGS devices, the second wave is already fast approaching. Although some of the newest additions appear to be modifications to some tried and tested procedures, their efficacy is yet to be proven and a lot more work is required to guide us through this maze.
TAKE HOME MESSAGE
-
The complexity, complications and follow-up burden of conventional glaucoma surgery has lead to a search for alternatives.
-
The early success of some MIGS procedures has encouraged a rapid expansion of new techniques.
-
All of these techniques are based on the principles of already existing operations.
-
Much work remains to be done in establishing their efficacy and safety, as well as the best candidates for surgery.
References
1. Olthoff CMG, Schouten JSAG, van de Borne BW, Webers CAB. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology 2005;112(6):953-61.
2. Kirwan JF, Lockwood AJ, Shah P, et al. Trabeculectomy in the 21st century: a multicenter analysis. Ophthalmology 2013;120(12):2532-9.
3. Craven ER, Katz LJ, Wells JM, Giamporcaro JE, iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg 2012;38(8):1339-45.
4. Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A randomized trial of a Schlemm’s canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Ophthalmology 2015;122(7):1283-93.
5. Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the Ocular Hypertension Treatment Study. Ophthalmology 2012;119(9):1826-31.
6. Batlle JF, Fantes F, Riss I, Pinchuk L, et al. Three-year follow-up of a novel aqueous humor microshunt. J Glaucoma 2016;25(2):e58-65.
7. Pérez-Torregrosa VT, Olate-Pérez Á, Cerdà-Ibáñez M, et al. Combined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisions. Arch Soc Espanola Oftalmol 2016;91(9):415-21.
8. Overby DR, Stamer WD, Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp Eye Res 2009;88(4):656-70.
9. Kaplowitz K, Bussel II, Honkanen R, et al. Review and meta-analysis of ab-interno trabeculectomy outcomes. Br J Ophthalmol 2016;100(5):594-600.
10. Seibold LK, Soohoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol 2013;155(3):524-9.
11. Grover DS, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy (GATT): thermal suture modification with a dye-stained rounded tip. J Glaucoma 2016;25(6):501-4.
12. Grover DS, Godfrey DG, Smith O, et al. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology 2014;121(4):855-61.
13. Grover DS, Smith O, Fellman RL, et al. Gonioscopy assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy for the treatment of primary congenital glaucoma and juvenile open angle glaucoma. Br J Ophthalmol 2015;99(8):1092-6.
14. Smith R. Nylon filament trabeculotomy. Comparison with the results of conventional drainage operations in glaucoma simplex. Trans Ophthalmol Soc N Z 1969;21:15-26.
15. Yan X, Li M, Chen Z, et al. Schlemm’s canal and trabecular meshwork in eyes with primary open angle glaucoma: a comparative study using high-frequency ultrasound biomicroscopy. PloS One 2016;11(1):e0145824.
16. Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of Schlemm’s canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: interim clinical study analysis. J Cataract Refract Surg 2007;33(7):1217-26.
17. Matlach J, Dhillon C, Hain J, et al. Trabeculectomy versus canaloplasty (TVC study) in the treatment of patients with open-angle glaucoma: a prospective randomized clinical trial. Acta Ophthalmol 2015;93(8):753-61.
18. Camras LJ, Yuan F, Fan S, et al. A novel Schlemm’s Canal scaffold increases outflow facility in a human anterior segment perfusion model. Invest Ophthalmol Vis Sci 2012;53(10):6115-21.
19. Gandolfi SA, Ungaro N, Ghirardini S, et al. Comparison of surgical outcomes between canaloplasty and Schlemm’s canal scaffold at 24 months’ follow-up. J Ophthalmol 2016:3410469.
20. Ab-interno canaloplasty - surgery that keeps its promise. 12 month case series review. Ellex; 2015.
www.ellex.com/wp-content/uploads/
sites/9/ABiC-Whitepaper-12-Months.pdf
Last accessed August 2016.
21. Berk TA, Tam DY, Werner L, et al. Electron microscopic evaluation of a gold glaucoma micro shunt after explantation. J Cataract Refract Surg 2015;41(3):674-80.
22. Vold S, Ahmed IIK, Craven ER, et al. Two-Year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology 2016 [Epub ahead of print].
23. Vernon SA, Koppens JM, Menon GJ, Negi AK. Diode laser cycloablation in adult glaucoma: long-term results of a standard protocol and review of current literature. Clin Experiment Ophthalmol 2006;34(5):411-20.
24. Lindfield D, Ritchie RW, Griffiths MF. ‘Phaco-ECP’: combined endoscopic cyclophotocoagulation and cataract surgery to augment medical control of glaucoma. BMJ Open 2012;2(3).
25. Aptel F, Béglé A, Razavi A, et al. Short- and long-term effects on the ciliary body and the aqueous outflow pathways of high-intensity focused ultrasound cyclocoagulation. Ultrasound Med Biol 2014;40(9):2096-106.
26. Aptel F, Charrel T, Lafon C, et al. Miniaturized high-intensity focused ultrasound device in patients with glaucoma: a clinical pilot study. Invest Ophthalmol Vis Sci 2011;52(12):8747-53.
27. Aptel F, Denis P, Rouland J-F, et al. Multicenter clinical trial of high-intensity focused ultrasound treatment in glaucoma patients without previous filtering surgery. Acta Ophthalmol 2016;94(5):e268–77.
28. Tan AM, Chockalingam M, Aquino MC, et al. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Experiment Ophthalmol 2010;38(3):266-72.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME