Hari Kaneshayogan interviews Salman Waqar, a Consultant Ophthalmologist with a keen interest in medical innovation. He is the inventor of the Waqar suture removal forceps, which combines suture cut and removal in one instrument. He has also created an injection guide with the potential to eliminate the need for a drape, calliper and speculum during intravitreal injections.
Salam Waqar.
What led you to create the Waqar suture removal forceps and intravitreal injection guide and how long did it take?
As doctors, our prime motivation is always to make procedures and treatments safer, and more comfortable, for patients. In the current financial climate, efficiency is desirable too, but this ultimately is also to the benefit of our patients. For me, all my innovations must pass the ‘family test’ – I would only ever recommend them to patients if I was happy and would want my family to have treatment the same way.
It was on this basis that both the intravitreal injection guide and suture removal forceps were designed. I observed that both procedures could be improved with simple instruments and got to putting ideas on paper. It has taken between three to four years from creation to availability for practical use.
What were the challenges you experienced when creating these devices and what did you learn along the way?
The main challenge was juggling this with the demands of training and family life. Each step threw up new questions requiring design modifications (the injection guide went through 11 such updates), suitable prototypes had to be made (3D printed or machine tooled), testing had to be organised on plastic / cadaveric and then human eyes, a commercial partner had to be found for manufacture and distribution, CE Marks and Food & Drug Administration (FDA) approval had to be achieved and, finally, hospital / patient approval had to be gained for local use.
The most important lesson I have learnt is to be clear from the start about your motivations. If you are passionate about developing something that will better the lives of your patients, the mentioned challenges will not be a trouble. If, however, the motivations are monetary, it is likely there will be disappointment as there is always the chance your innovation might not make it past all the hurdles. Translating your idea to industry production may not always be possible but accept failure as a part of the process and use it as a learning opportunity for your next great idea.

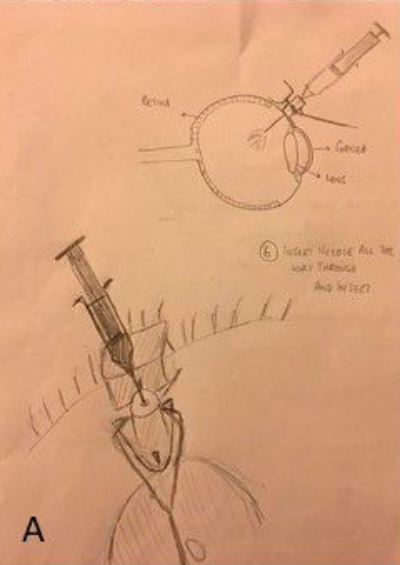
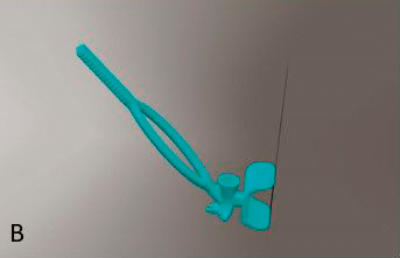
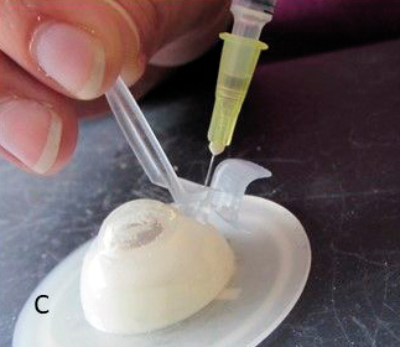
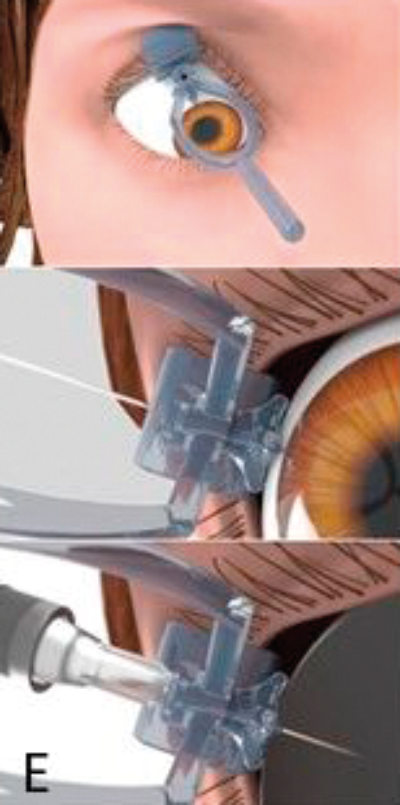
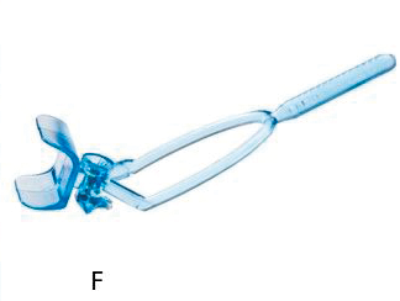
Figure 1: The development process: (A) Hand drawn diagrams; (B) CAD design; (C) Testing 3D printed prototype on plastic eye;
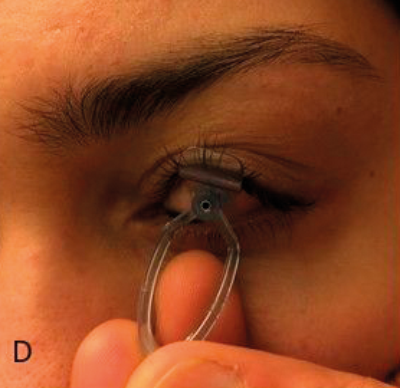
(D) Device placement on volunteer eye without injection; (E) Procedure animations; (F) Final instrument; (G) Patient injection.
What resources exist within the NHS to help inventors with patenting, design and production?
I have been very lucky that there exists a regional innovation pathway in the South West Peninsula. Initially within the remit of the local Academic Health Science Network (AHSN), this is now run independently as a collaboration between the local research and development (R&D) departments. This pathway was able to provide much needed expertise in all areas ranging from computer-aided designs (CAD) designs, 3D printing and intellectual property (IP), to negotiating contracts with industry partners. The NHS is a hot-bed of ideas and it is to the organisation’s benefit to harness these ideas in-house.
Was it difficult to gain ethical approval for usage on real patients?
Any new invasive device (such as the injection guide) first requires a CE Mark and FDA approval (in this case a 510-k exemption) for which I received guidance from both my Trust and our commercial partners (BVI Malosa). I then had to apply for Clinical Effectiveness Group (CEG) approval within the Trust. This was granted on the provision that the procedure would only be performed after detailed patient consent. Details of all patients were kept for audit purposes and regular reports were required by the CEG. It was delightful to note that none of our patients refused participation, the overwhelming reply being that they were happy to help if it would improve things for others.
As the suture removal instrument is non-invasive, we were able to go ahead and use it simply by acquiring a CE Mark.
I have an idea for a medical device what do I do next?
Congratulations on taking the first step! I would consider the following important points:
(a) As an NHS employee, your employer holds the IP rights to any idea that is related to your routine work. Therefore, it is important that your Trust is the first port of call.
(b) Write down or draw your idea and sign / date it immediately. This may sound a bit over the top, but you can also post it to yourself as the post office date on the letter becomes legal proof. If someone else develops a similar idea, whoever can prove they came up with the idea first will own the IP rights.
(c) Tempting as it may be, resist the impulse to discuss widely or present at meetings until the idea is fully developed or a patent applied for. First and foremost, discuss with the R&D department who will then advise you on the best way forward whilst also protecting the IP. All NHS employees are contractually obliged to keep such information confidential, so you could run the idea past a trusted senior colleague, but best to always have documentation of the discussion (e.g. email trail).
What are the common pitfalls to avoid in the process of medical innovation?
The main pitfall is not taking adequate steps to protect your claim to the IP rights. This can cause difficulties when approaching potential industry partners and in the form of legal challenges once a patent has been acquired and the product comes to market. IP laws can be notoriously complex, and it is very advisable to get your R&D department involved at the outset.
Which resources would you recommend to find out more about innovation?
Local R&D newsletters, drop-in sessions and websites are a good starting point. In addition, regional AHSN publish annual reports with details of useful contacts and past projects.
COMMENTS ARE WELCOME