Consent is a hot topic at the moment and the publication of the GMC Guidance on Consent [1] has rightfully refocussed our attention on it. Consent practices vary wildly and have been the subject of many of these surveys.
Once we have obtained a signature on the pre-requisite form as an acknowledgment that the patient agrees they have given informed consent to proceed with an intervention, how long should that consent last? If the patient is consenting for a series of treatments, should they sign a consent each and every time? Can one consent form, and therefore one consent discussion, cover all of their treatments?
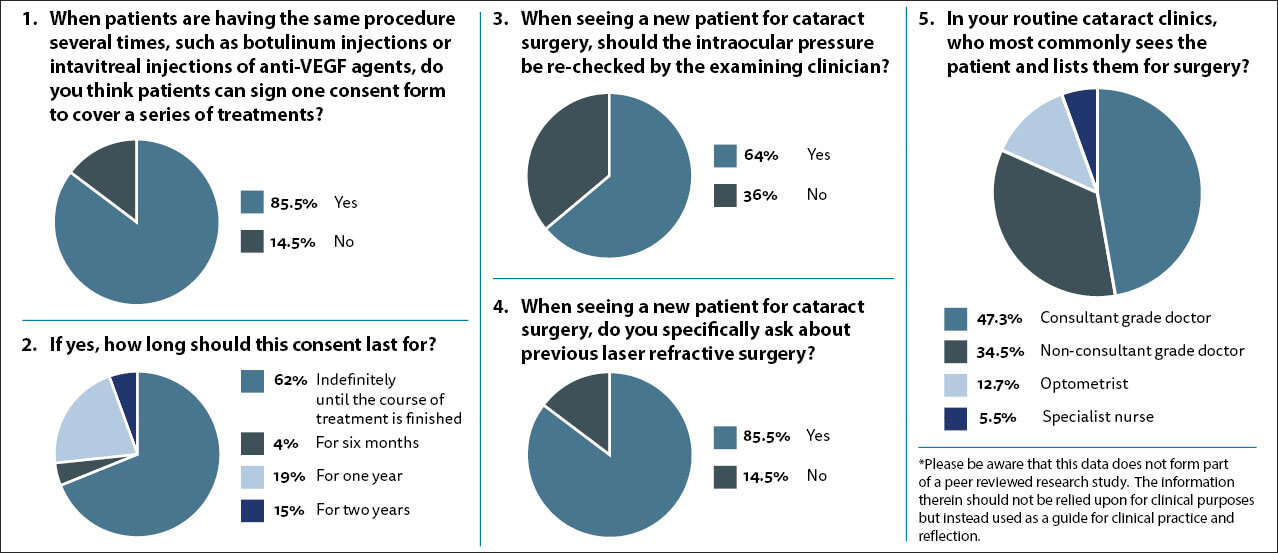
When faced with this question the vast majority of you (85.5%) felt that one consent form can cover a series of treatments, such as intravitreal or botulinum toxin injections. I feel this is reasonable, however, it is always worth reminding the patient of the rationale behind their treatment and giving them the opportunity to ask further questions if they wish.
When asked how long this consent should last for there was some variance in response with 62% of respondents indicating that the consent could last for the entire duration of their treatment, while 4%, 19% and 15% of you felt that the consent could last for six months, one year and two years respectively. As far as I am aware there is no specific guidance and to me placing an arbitrary duration seems without evidence base. I think we need to focus on the reason for the consent process which is to give the patient autonomy over their bodies and allow them to make informed choices about treatment.
I believe that the consent is valid for as long as there is no change in the patient and their ability to comprehend / recall the information previously provided to them AND there is no material change in the risk-benefit profile of the treatment they are agreeing to.
Taking the intravitreal injections of anti-vascular endothelial growth factor (VEGF) scenario, the patient embarking on their first intravitreal injection for wet age-related macular degeneration (AMD) with 6/12 vision and a clear potential benefit from a course of anti-VEGF therapy will, on the balance of probabilities, be perfectly happy to accept the one in 1500 chance of infective endophthalmitis. Leap forward 18 months and the scenario the patient finds themselves in may be very different. If their vision is now counting fingers and there is persistent leakage despite 15 intravitreal injections with a poor prognosis for any visual improvement, their attitude to the one in 1500 risk may be different. The risk-benefit profile may remain favourable for treatment, however, the original consent discussion no longer applies. In these circumstances it may be wise to redo the consent discussion and get another signature to cement that further agreement.
I am regularly faced by clinical records which appear very sparsely populated. I often wonder at what the minimum data set collected by clinicians for conditions such as cataracts should be. Clearly, there are some investigations which are not necessary. While gonioscopy is vital for a patient presenting with raised intraocular pressure (IOP), it is not routinely necessary for a patient presenting with a cataract. When I see a cataract patient in the clinic, I routinely recheck their IOP even if the referring optometrist checked it and it was normal. I have detected undiagnosed raised IOPs on a number of occasions, but it is admittedly very rare and in each of those cases I do not think I deferred the surgery or commenced treatment. When asked whether the IOP should be rechecked, two-thirds of respondents felt it should be and one-third felt it was not necessary. My feeling remains that is it part of the baseline clinical examination and if we did detect an unexpected significantly raised IOP it may have implications for our surgery.
Laser refractive surgery has been around for some time now and the 60-year-olds presenting to us with age-related cataracts may have had laser assisted in situ keratomileusis (LASIK) or photorefractive keratectomy (PRK) 20 years prior. They may not have an understanding that the laser treatment they had in their 40s would have any bearing on their potential operation now. We need to be asking the specific question, as there is a material risk of avoidable harm, in the form of unexpected refractive error, if the fact is missed and the wrong biometry is used. Eighty-five percent of you agree and I would urge the remainder to make it part of their normal practice to ask the question.
With the new consent guidance there has been some concern over whether the practice of pooled operating lists is compatible with appropriate consent practice. The concern is whether consent is valid if it is taken by one clinician when a different clinician is operating. Furthermore, if the clinician who has the consent discussion does not operate themselves, can they truly explain the ins and outs of the procedure and the material risks involved? I think with appropriate training they can, and it is not an issue which concerns me, but I believe there should be some formal validation.
When asked who commonly sees the patient in clinic, almost half responded that it was the consultant, a third said it was a non-consultant grade doctor, while in 18.2% the patient was seen by an optometrist or specialist nurse. I can see the benefits in cost-effectiveness and facilitating volumes / throughput for using allied professionals and I am firm believer in utilising the skills and abilities of our allied professionals, however, I believe that formal training in the procedure-specific consent process and the supervision / ability to call upon a doctor for advice would be ideal. As services evolve in the capacity stretched circumstances we find ourselves in, the models of care will have to be reviewed and optimised.
Reference
1. General Medical Council. Decision making and consent.
https://www.gmc-uk.org/ethical-guidance/
ethical-guidance-for-doctors/decision-making-and-consent
(Last accessed November 2021).
COMMENTS ARE WELCOME






